Published TCIMAIL newest issue No.200
Maximum quantity allowed is 999

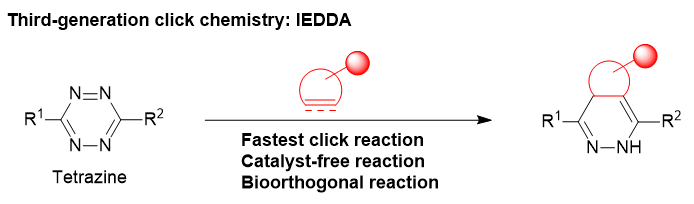
The 1,2,4,5-tetrazines are known to undergo the Strain-Promoted Inverse Electron-Demand Diels-Alder Reaction (SPIEDAC) with strained C-C multiple bonds and are gaining attention as third-generation click reactions. This is a novel method that overcomes the limitations of traditional click reactions, enabling more efficient and specific bonding. Additionally, this reaction meets the criteria for bioorthogonal reactions (fast, selective, biocompatible, catalyst-free) and is being recognized as an innovative technology across various fields such as protein labeling, imaging, and pharmaceutical development.1-4)
Characteristics of Third-Generation Click Reactions
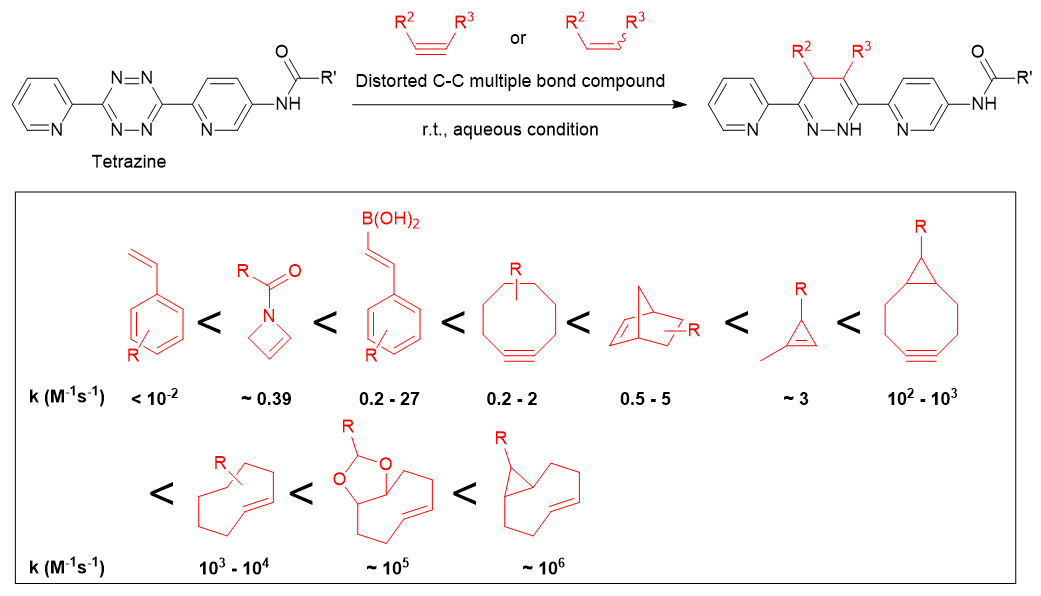
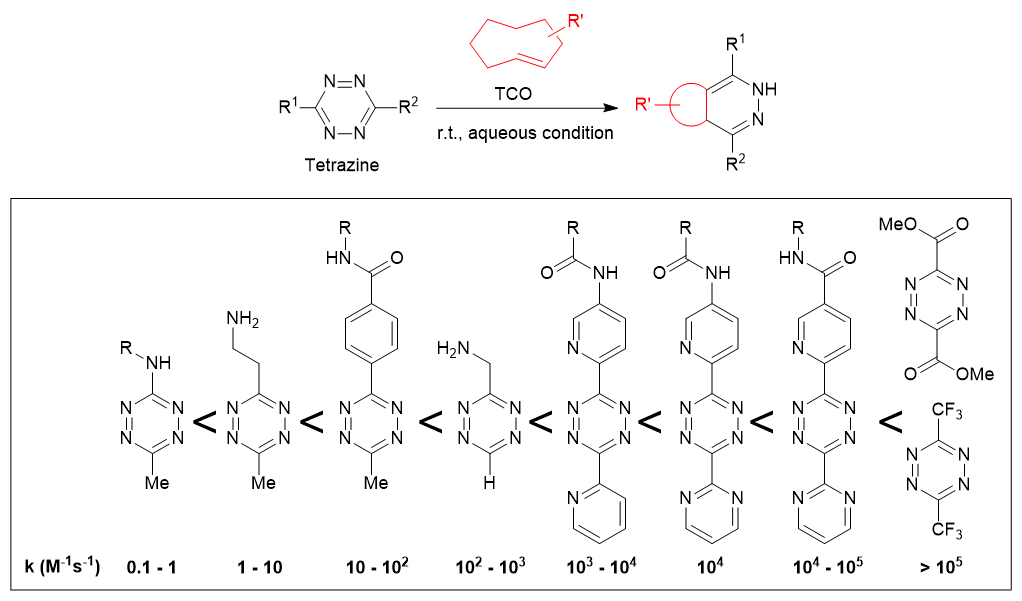
A well-known reaction involving 1,2,4,5-tetrazines is their reaction with TCO (trans-cyclooctene), which occurs at a rate of k = 1 - 106 M-1s-1. This rate is significantly faster compared to the second-generation click reaction (strain-promoted azide-alkyne cycloaddition, SPAAC), which has a reaction rate of approximately k = 10-2 - 10-1 M-1s-1.
It has been suggested that the reaction proceeds much more rapidly when the tetrazine is electron-deficient and has less steric hindrance, and when the reaction partner has a highly strained C-C multiple bond.5)
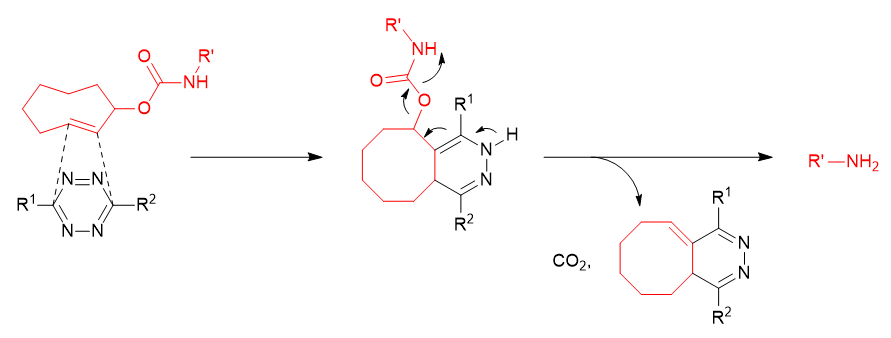
Furthermore, the reaction product of carbamate-type TCO* (2-TCO) derivatives with 1,2,4,5-tetrazines has been reported to undergo a dissociation reaction, releasing an amine. This has raised expectations for its role as a prodrug via a "click-to-release" reaction mechanism.6-9)
Based on these characteristics, we offer a wide range of hetero-bifunctional crosslinkers containing 1,2,4,5-tetrazine. Please use them in conjunction with our TCO derivatives and related products.