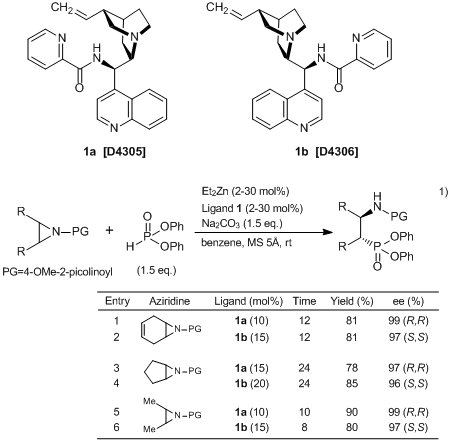
Nakamura
et al. have developed the heteroarenecarbonyl cinchona alkaloid catalysts,
N-(9-deoxy-epi-cinchonin-9-yl)picolinamide (
1a) and
N-(9-deoxy-epi-cinchonidin-9-yl)picolinamide (
1b), and reported the enantioselective desymmetrization of aziridines with phosphites using
1. According to their results, various aziridines are converted into optically active β-aminophosphonic acids in high yields with high enantioselectivities by using
1 and Et
2Zn as catalysts. In this approach, both enantiomers are directly synthesized by using either
1a and
1b. Obtained optically active β-aminophosphonic acids and their derivatives can be used as biologically active substances and chiral ligands.