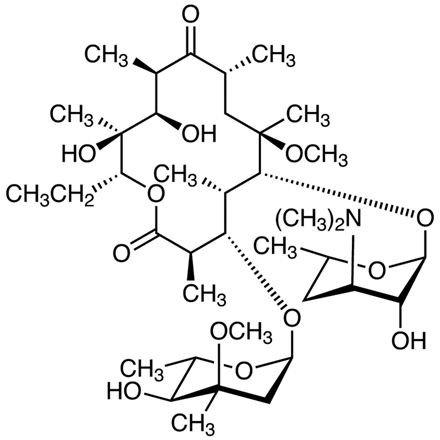
Macrolide antibiotics are classified by the size of the macrocyclic lactone ring of aglycone as 14-, 15- or 16-membered ring macrolides. Macrolides are characterized by similar chemical structures, mechanisms of action and resistance, but vary in the different pharmacokinetic parameters, and spectrum of activity. Clarithromycin (abbrev: CAM) is chemically synthesized by substituting a methyl group for the C-6 hydroxyl group of erythromycin [
E0751]. This substitution creates a more acid-stable antimicrobial and prevents the degradation of the erythromycin base to the hemiketal intermediate. CAM is primarily metabolized by cytochrome P450 (CYP) 3A isoenzymes to an active metabolite, 14-hydroxyclarithromycin. CAM has activity against a wide range of Gram-positive bacteria including
Streptococcus and
Staphylococcus, and limited activity against Gram-negative bacteria, similar to that of erythromycin. CAM demonstrates greater
in vitro activity than erythromycin against certain pathogens including
Hemophilus influenza and
Helicobacter pylori. However, bacterial strains resistant to erythromycin are also generally resistant to clarithromycin. CAM inhibits protein synthesis in susceptible organisms by binding to specific nucleotides in 23S rRNA within the 50S subunit of the bacterial ribosome. CAM and macrolides also shows gastrointestinal motor stimulating activity as a motilin-agonist, and anti-inflammatory or immunomodulatory activity.