Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
Merci de sélectionner la quantité
A Safe Cyano(nitro)methylation Reagent
No.170(July 2016)
Nitroacetonitrile (NAN) is useful as a cyano(nitro)methylation reagent for synthetic chemistry because it has a highly-acidic methylene connected with reactive cyano and nitro functionalities. However, NAN has an explosive nature. Therefore, a safe alternative for cyano(nitro)methylation reagent is required.
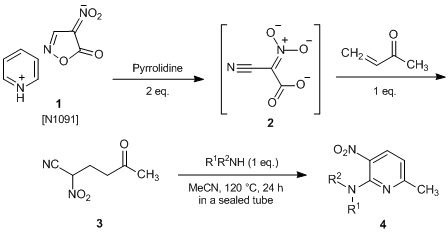
The pyridinium salt of nitroisoxazolone 1, which has been developed by Nishiwaki et al., undergoes a ring opening reaction quantitatively under mild conditions upon treatment with two equivalents of organic base and is converted to cyano-aci-nitroacetate 2, which works as a synthetic equivalent of NAN.1) 2 has high solubility in organic solvents whether polar or non-polar. Hence, it is useful as a safe and easy-to-handle cyano(nitro)methylation reagent for synthesis of versatile polyfunctionalized compounds.
For example, bifunctional pyridine 4, which possesses vicinally substituted nitro group and amino group, is formed when the Michael adduct 3, which is obtained by adding a vinyl ketone to generated 2, reacts with amines.2) Also, the syntheses of polyfunctionalized heterocyclic compounds such as isoxazolines, isoxazoles, dihydropyridines, and naphthyridines by using 2 have been reported.2-4)
In addition, products obtained using cyano-aci-nitroacetate 2 derived from 1 can be introduced to versatile frameworks because they possess a cyano group and a nitro group.
References
- 1)Ring opening reaction of the pyridinium salt of 4-nitro-3-isoxazolin-5-one
- 2)Safe cyano(nitro)methylating reagent – Michael addition of cyano-aci-nitroacetate leading to δ-functionalized α-nitronitriles
- 3)One-pot synthesis of polyfunctionalized isoxazol(in)es
- 4)Synthesis of vicinally functionalized 1,4-dihydropyridines and diazabicycles via a pseudo-intramolecular process
- 5)Review
- H. Asahara, N. Nishiwaki, Oreo Saiensu 2015, 15, 165.
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

