Published TCIMAIL newest issue No.199
Maximum quantity allowed is 999
Bitte wählen Sie die Menge aus.
Heteroarenecarbonyl Cinchona Alkaloid Catalysts
No.159(October 2013)
Nakamura et al. have developed the heteroarenecarbonyl cinchona alkaloid catalysts 1, and reported the enantioselective desymmetrization of aziridines with phosphites using 1.1) According to their results, various aziridines are converted into optically active β-aminophosphonic acids in high yields with high enantioselectivities by using 1 and Et2Zn as catalysts. In this approach, both enantiomers are directly synthesized by using either 1a and 1b. Obtained optically active β-aminophosphonic acids and their derivatives can be used as biologically active substances and chiral ligands.
Nakamura et al. have also reported the cinchona alkaloid amide/copper(II) catalyzed diastereo- and enantioselective vinylogous Mannich reaction of ketimines.2) This article has been chosen as a "Hot Paper" by the editors of "Angewandte Chemie" for its importance.
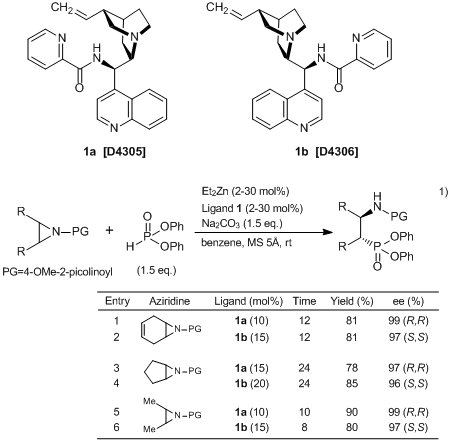
Typical Procedure: Enantioselective Desymmetrization of Aziridines with Phosphites (Entry 1)
MS 5Å (100 mg) and Na2CO3 (32 mg, 0.30 mmol) are heated with heat-gun, and it is heated at 140 °C under reduced pressure for 1 h. To a suspension of 1a (8.0 mg, 0.02 mmol), MS 5Å and Na2CO3 in benzene (1.0 mL) is added Et2Zn (1.0 M in toluene, 20 μL, 0.02 mmol) and stirred for 10 min. A solution of aziridines (0.20 mmol) and diphenyl phosphite (58 μL, 0.30 mmol) in benzene (0.5 mL) is added to the reaction mixture and is stirred for 12 h. Then the reaction mixture is diluted with AcOEt and filtered through a celite pad. The volatile compounds are removed under reduced pressure and the crude product is purified by silica gel column chromatography (Hexane : AcOEt=1 : 1) to give the desired (R,R)-product. The (S,S)-product is obtained by using 1b instead of 1a.
MS 5Å (100 mg) and Na2CO3 (32 mg, 0.30 mmol) are heated with heat-gun, and it is heated at 140 °C under reduced pressure for 1 h. To a suspension of 1a (8.0 mg, 0.02 mmol), MS 5Å and Na2CO3 in benzene (1.0 mL) is added Et2Zn (1.0 M in toluene, 20 μL, 0.02 mmol) and stirred for 10 min. A solution of aziridines (0.20 mmol) and diphenyl phosphite (58 μL, 0.30 mmol) in benzene (0.5 mL) is added to the reaction mixture and is stirred for 12 h. Then the reaction mixture is diluted with AcOEt and filtered through a celite pad. The volatile compounds are removed under reduced pressure and the crude product is purified by silica gel column chromatography (Hexane : AcOEt=1 : 1) to give the desired (R,R)-product. The (S,S)-product is obtained by using 1b instead of 1a.
References
- 1)Cinchona alkaloid amides/dialkylzinc catalyzed enantioselective desymmetrization of aziridines with phosphites
- 2)Cinchona alkaloid amide/copper(II) catalyzed diastereo- and enantioselective vinylogous Mannich reaction of ketimines with siloxyfurans
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

