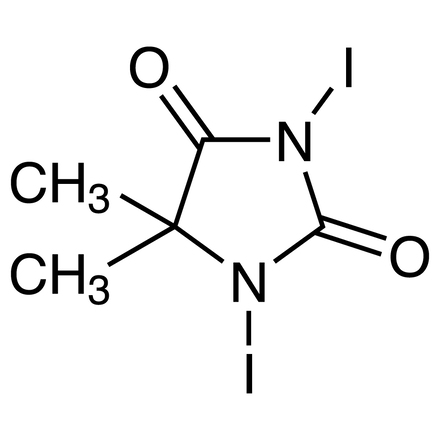
An efficient synthesis of 2-substituted-2-oxazolines using 1,3-diiodo-5,5’-dimethylhydantoin (DIH)
Typical procedure: To a solution of p-tolualdehyde (120.2 mg) in tert-butanol (10 mL) is added (R)-(–)-2-phenylglycinol (205.8 mg). The mixture is stirred at rt under argon for 30 min, and then DIH (569.9 mg) is added and the mixture is stirred at 50 °C. After 24 h, the mixture is quenched with saturated aqueous Na2CO3 until the color of I2 has almost disappeared, and is extracted with CHCl3 (3 x 15 mL). The combined organic layers are washed with aqueous K2CO3 (10 mL) and brine (10 mL), and dried over Na2SO4. After filtration, the mixture is evaporated and the residue is purified by chromatography on silica gel (eluent: EtOAc) to give (4R)-2-(4’-methylphenyl)-4-phenyl-2-oxazoline (201.7 mg, 85 %) as an oil.
Typical Procedure:
2) Sulfuric acid (2 mL) is added under stirring and cooling to a solution of anisole (1.1 g, 10 mmol) in ethanol (15 mL), and DIH (
1; 1.9 g, 5 mmol) is then added in 3 portions over a period of 2-3 min. When the reaction is complete, the mixture is diluted with 3% solution of Na
2SO
3 (50 mL), and the precipitate is filtered off, washed with water, dried, and recrystallized from appropriate solvent. Yield of 4-iodoanisole 97%.