Published TCIMAIL newest issue No.199
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 19999-87-2 | Product Number: B3534
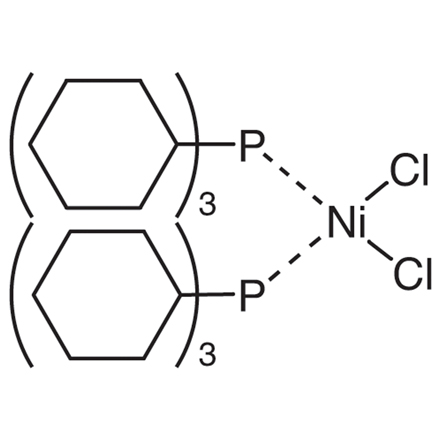
Bis(tricyclohexylphosphine)nickel(II) Dichloride
Purity: >95.0%(T)
Synonyms:
- Dichlorobis(tricyclohexylphosphine)nickel(II)
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 1G |
€31.00
|
2 | ≥40 |
|
| 5G |
€124.00
|
1 | 14 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | B3534 |
Purity / Analysis Method 
|
>95.0%(T) |
| Molecular Formula / Molecular Weight | C__3__6H__6__6Cl__2NiP__2 = 690.46 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
Packaging and Container 
|
1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 19999-87-2 |
| Reaxys Registry Number | 4935620 |
| PubChem Substance ID | 125307309 |
| MDL Number | MFCD11973802 |
Specifications
| Appearance | Yellow to Amber to Dark red powder to crystal |
| Purity(Chelometric Titration) | min. 95.0 % |
| NMR | confirm to structure |
Properties (reference)
| Melting Point | 227 °C |
GHS
| Pictogram |


|
| Signal Word | Danger |
| Hazard Statements | H302 : Harmful if swallowed. H372 : Causes damage to organs through prolonged or repeated exposure. H317 : May cause an allergic skin reaction. H334 : May cause allergy or asthma symptoms or breathing difficulties if inhaled. H350 : May cause cancer. |
| Precautionary Statements | P260 : Do not breathe dust. P201 : Obtain special instructions before use. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection/ hearing protection. P342 + P311 : If experiencing respiratory symptoms: Call a POISON CENTER/doctor. P308 + P313 : IF exposed or concerned: Get medical advice/ attention. P304 + P340 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. |
Related Laws:
Transport Information:
| HS Number | 2931599090 |
Application
Nickel Complexes for the Cross-Coupling Reactions Using Alkoxy Groups as Leaving Groups

References
- Activation of Grignard reagents by transition-metal complexes. A new and simple synthesis of trans-stilbenes and polyphenyls
- Selective carbon-carbon bond formation by cross-coupling of Grignard reagents with organic halides. Catalysis by nickel-phosphine complexes
- Nickel-Catalyzed Cross-Coupling of Aryl Grignard Reagents with Aromatic Alkyl Ethers: An Efficient Synthesis of Unsymmetrical Biaryls
Application
Nickel-Catalyzed Cross-Coupling of Aryltrimethylammonium Iodides with Organozinc Reagents

Typical procedure (Ar = Ph, R = 4-CH3Ph): Trimethylphenylammonium iodide (132 mg), bis(tricyclohexylphosphine)nickel(II) dichloride (6.9 mg) and NMP (1.5 mL) are added to a Schlenk tube. To the stirring mixture, 4-CH3C6H4ZnCl solution (1.5 mL, 1.5M solution in THF) is added by a syringe. The reaction mixture is stirred at 90 °C for 8 h and then cooled to room temperature. Water (10 mL) and several drops of acetic acid are successively added. The resulting mixture is extracted with diethyl ether (3 x 10 mL) and the extract is dried over Na2SO4, filtered and concentrated. The residue is purified by column chromatography on silica gel (eluent: petroleum ether or petroleum ether/ethyl acetate 60:1) to give 4-methylbiphenyl (76.5 mg, 91 %).
References
PubMed Literature
Documents
Product Articles
[TCIMAIL No.148] A Unique and Efficient Ni Catalyst for Cross-Coupling Reactions[Research Articles] Nickel-Catalyzed Cross-Coupling of Aryltrimethylammonium Iodides with Organozinc Reagents
[Product Highlights] Nickel Complexes for the Cross-Coupling Reactions Using Alkoxy Groups as Leaving Groups
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





