Published TCIMAIL newest issue No.199
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 9041-93-4 | Product Number: B3972
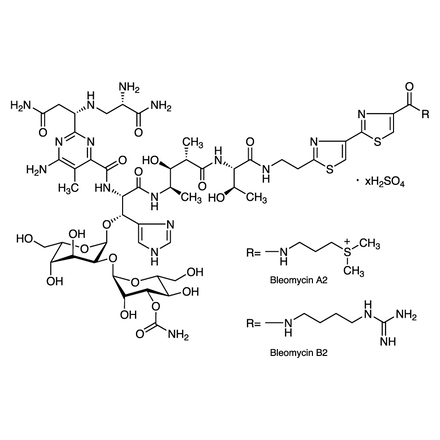
Bleomycin Sulfate (mixture)
Purity: >85.0%(HPLC)
Synonyms:
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 10MG |
€141.00
|
2 | ≥80 |
|
| 50MG |
€550.00
|
2 | ≥100 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
Supplemental Product Information:
This product has not been tested for potency and is guaranteed for chemical purity.
| Product Number | B3972 |
Purity / Analysis Method 
|
>85.0%(HPLC) |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Hygroscopic,Heat Sensitive |
Packaging and Container 
|
10MG-Glass Bottle with Plastic Insert (View image), 50MG-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 9041-93-4 |
Related CAS RN 
|
11056-06-7 |
| PubChem Substance ID | 253659941 |
| Merck Index (14) | 1318 |
| MDL Number | MFCD00070310 |
Specifications
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min.85.0 area% (Sum of A2,B2) |
| BleomycinA2 | 55.0 to 70.0 area% |
| BleomycinB2 | 25.0 to 32.0 area% |
Properties (reference)
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H351 : Suspected of causing cancer. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P202 : Do not handle until all safety precautions have been read and understood. P201 : Obtain special instructions before use. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection/ hearing protection. P308 + P313 : IF exposed or concerned: Get medical advice/ attention. P405 : Store locked up. |
Related Laws:
| EC Number | 232-925-2 |
| RTECS# | EC5991990 |
Transport Information:
| HS Number | 2941900000 |
Application
Quorum sensing inhibitor
Reference
- The Role of Drug Repurposing in the Development of Novel Antimicrobial Drugs: Non-Antibiotic Pharmacological Agents as Quorum Sensing-Inhibitors
Application
Adeno-associated virus vector transduction enhancer
Bleomycin increases transduction by adeno-associated virus (AAV) vectors.1)
References
- 1) Identification and validation of small molecules that enhance recombinant adeno-associated virus transduction following high-throughput screens
Application
Reagent for pneumonitis model
References
- Effect of lecithinized-superoxide dismutase on the interstitial pneumonia model induced by bleomycin in mice
- Regulation of type II alveolar epithelial cell proliferation by TGF-beta during bleomycin-induced lung injury in rats
- Production of superoxide and nitric oxide by alveolar macrophages in the bleomycin-induced interstitial pneumonia mice model
- Dual effect of AMD3100, a CXCR4 antagonist, on bleomycin-induced lung inflammation
- Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model
- In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats
- Effects of phosphodiesterase 4 inhibition on bleomycin-induced pulmonary fibrosis in mice
Application
Reagent for pulmonary fibrosis model
References
- Modeling pulmonary fibrosis with bleomycin
- Pulmonary fibrosis: searching for model answers
Application
Bleomycin: A Glycopeptide Antitumor Antibiotics Agent
Bleomycin, a glycopeptide antitumor antibiotic agent, was first discovered in 1966 when the Japanese bacteriologist Umezawa found anticancer activity while screening culture filtrates of Streptomyces verticillus. Activated bleomycin (Fe-bleomycin-O2 complex), the active species, forms in the reaction of bleomycin with Fe(II) and O2. It also forms in the reaction of bleomycin with Fe(III) and peroxide, or bleomycin with superoxide and either Fe(III) or Fe(II). The activated bleomycin produces DNA strand break at 3'-4' bond in a deoxyribose sugar moiety. The mechanism of this bond cleavage reaction and the nature of the active oxidizing species are still open issues.
References
- Mechanisms of bleomycin-induced DNA degradation (a review)
- Cleavage of Nucleic Acids by Bleomycin (a review)
- Bleomycin: New Perspectives on the Mechanism of Action (a review)
- Antitumor Antibiotics: Bleomycin, Enediynes, and Mitomycin (a review)
- Direct Hydrogen-Atom Abstraction by Activated Bleomycin: An Experimental and Computational Study
PubMed Literature
Documents
Product Articles
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





