In the field of biochemical research, buffering agents perform a very important function. The Tris buffers widely used today have a primary amino group and they are known to frequently cause inhibition problems in biological systems. Furthermore sufficient buffering power cannot be obtained under pH7.5.
Good and co-workers have developed buffers to overcome the above-noted defects and their superiority has been indicated by the Hill reactions. These buffers are referred to as Good’s Buffers being named after the inventor.
[Characteristics] 1) Acid dissociation constant p
Ka is between 6~8.
2) High water solubility.
3) Low penetration through biomembranes.
4) Low base effect toward biological systems.
5) p
Ka is less affected by concentration, temperature and ion composition.
6) Low complexation ability with metal ions.
7) Chemically stable.
8) Low in absorption of visible and ultra-violet rays.
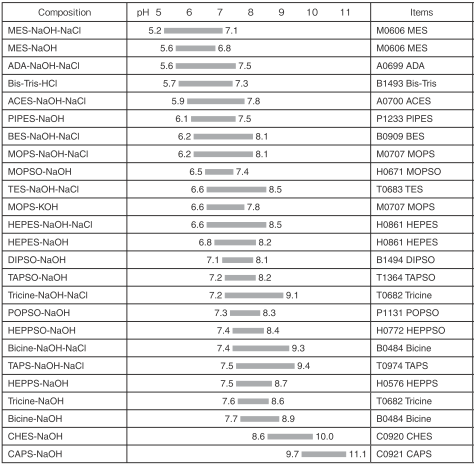
Composition and pH range