Dimethyl(2-pyridyl)silyl Compounds
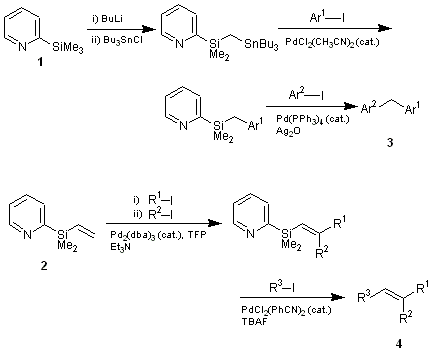
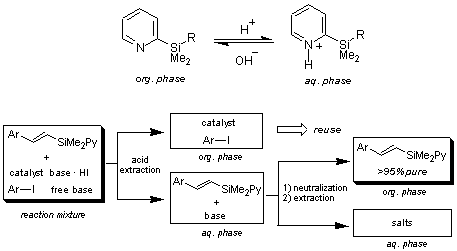
Yoshida and coworkers have demonstrated that pyridylsilane 1 and 2 can be reacted with tributylstannyl chloride to form a gem-dimetalmethane as a platform for various cross-coupling reactions. In their demonstration, tributylstannyl group is first incorporated into pyridylsilane 1 to make a gem-dimetal, which is then convereted to diarylmethanes 3 by the Stille reaction, followed by the Hiyama reaction.1) In the case of pyridylsilane 2, combination of the Heck reaction and the Hiyama reaction produced multisubstituted olefins 4.2) 1 and 2 can also be utilized in the synthesis of certain alcohols3) and cyclopentenones.4) The additional benefit of using the dimethyl(2-pyridyl)silyl compounds are that they can also be utilized as phase tag as shown in the scheme below. A simple acid-base extraction enables an easy separation/purification of the products feasible.5)
References
- 1)Synthesis of diarylmethanes
- 2)2-Pyridyldimethyl(vinyl)silane as a versatile platform for olefin synthesis
- 3)(2-Pyridyldimethylsilyl)methyl lithium as a novel hydroxymethylating reagent
- 4)Synthesis of cyclopentenones
- 5)Dual role of 2-pyridyldimethylsilyl group as a directing group and as a phase tag
- 6)Reviews
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.