Published TCIMAIL newest issue No.199
Maximum quantity allowed is 999
* Stock available in Belgium: Shipment on the same day
* Stock available in Japan: Please check the Shipping Simulation for estimated shipments. (excludes regulated items and dry ice shipments)
| Product Number | R0069 |
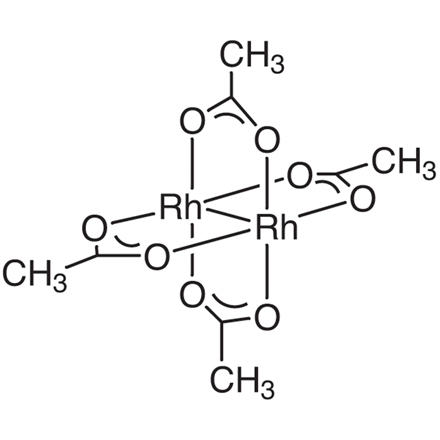
| Molecular Formula / Molecular Weight | C__8H__1__2O__8Rh__2 = 441.99 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
Packaging and Container 
|
100MG-Glass Bottle with Plastic Insert (View image), 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 15956-28-2 |
| Reaxys Registry Number | 14201302 |
| PubChem Substance ID | 87575627 |
| MDL Number | MFCD00003538 |
| Appearance | Green to Dark green powder to crystal |
| Elemental analysis(Carbon) | 20.50 to 22.70 % |
| Melting Point | 240 °C |
| EC Number | 240-084-8 |
| RTECS# | VI9361000 |
| HS Number | 2843909000 |


The requested SDS is not available.
Please Contact Us for more information.
A sample C of A for this product is not available at this time.

The requested analytical chart is not available. Sorry for the inconvenience.