Maintenance Notice (12:30 AM - 4:00 AM June 8, 2025): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.198
Maximum quantity allowed is 999
| Size | Unit Price | Belgium | Japan* |
|---|---|---|---|
| 5G |
€28.00
|
6 | ≥100 |
| 25G |
€98.00
|
10 | ≥100 |
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | T1292 |
| Purity / Analysis Method | >98.0%(T) |
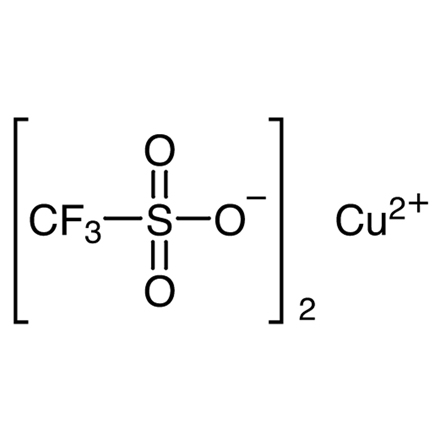
| Molecular Formula / Molecular Weight | C__2CuF__6O__6S__2 = 361.67 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Hygroscopic |
| CAS RN | 34946-82-2 |
| Reaxys Registry Number | 4028198 |
| PubChem Substance ID | 87577063 |
| MDL Number | MFCD00077492 |
| Appearance | White to Gray to Dark blue powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
| Solubility in water | Soluble |
| Pictogram |

|
| Signal Word | Danger |
| Hazard Statements | H314 : Causes severe skin burns and eye damage. |
| Precautionary Statements | P260 : Do not breathe dust. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection/ hearing protection. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. |
| EC Number | 252-300-8 |
| UN Number | UN1759 |
| Class | 8 |
| Packing Group | III |
| HS Number | 2904100090 |



The requested SDS is not available.
Please Contact Us for more information.
A sample C of A for this product is not available at this time.
The requested analytical chart is not available. Sorry for the inconvenience.