Published TCIMAIL newest issue No.200
Maximum quantity allowed is 999
* Stock available in Belgium: Shipment on the same day
* Stock available in Japan: Please check the Shipping Simulation for estimated shipments. (excludes regulated items and dry ice shipments)
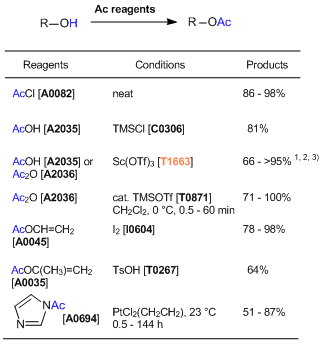
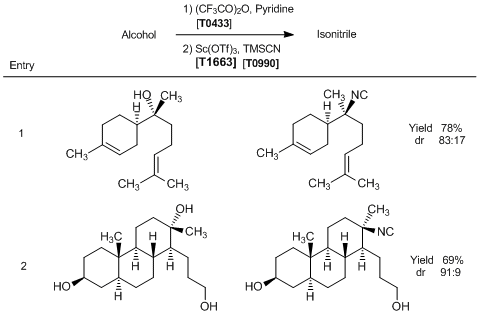
| Product Number | T1663 |
Purity / Analysis Method 
|
>98.0%(T) |
| Molecular Formula / Molecular Weight | C__3F__9O__9S__3Sc = 492.15 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Hygroscopic |
Packaging and Container 
|
1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 144026-79-9 |
| Reaxys Registry Number | 8510151 |
| PubChem Substance ID | 87577404 |
| MDL Number | MFCD00192433 |
| Appearance | White to Almost white powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
| Solubility in water | Soluble |
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| HS Number | 2846903000 |
The requested SDS is not available.
Please Contact Us for more information.
A sample C of A for this product is not available at this time.

The requested analytical chart is not available. Sorry for the inconvenience.