Application
Protection of Diols
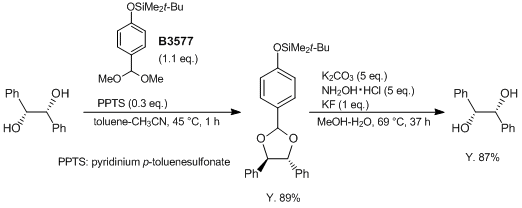
Typical Procedure
(Protection of Diols):
A 500-mL, oven-dried, three-necked round-bottomed flask equipped with a reflux condenser (fitted with an argon gas inlet), a teflon-coated magnetic stir bar, a rubber septum, and a thermocouple is charged with (R,R)-(+)-1,2-diphenyl-1,2-ethanediol (6.43 g, 30.0 mmol), toluene (96 mL), CH3CN (32 mL), and tert-butyl[4-(dimethoxymethyl)phenoxy]dimethylsilane (9.27 g, 32.8 mmol). After stirring the solution for 3 min, PPTS (2.26 g, 9.0 mmol) is added. The reaction mixture is heated in a 50 °C oil bath (internal temperature: 45 °C) and is stirred for 1 h. The mixture is cooled to ambient temperature and then is poured into sat. aq. NaHCO3 solution (100 mL) in a 1-L separatory funnel, and the suspension is extracted with EtOAc (3 × 100 mL). The combined organic extracts are washed with sat. aq. NaCl solution (2 × 100 mL), then are dried over anhydrous Na2SO4 (20 g), filtered, and concentrated on a rotary evaporator under reduced pressure to give a yellow, viscous oil. The residue is purified by silica gel column chromatography (hexane : CH2Cl2 = 7 : 3) to give a colorless, viscous oil (12.33 g) which is triturated with MeOH to yield the product as a white solid (11.51 g, 89%).
(Deprotection): A 500-mL, three-necked round-bottomed flask equipped with a reflux condenser (fitted with an argon inlet), a teflon-coated magnetic stir bar, a rubber septum, and a thermocouple is charged with the protected compound (10.8 g, 25.0 mmol), K2CO3 (17.3 g, 125 mmol), MeOH (200 mL), and water (50 mL). After addition of KF (1.46 g, 25.0 mmol) and stirring for 5 min followed by addition of NH2OH.HCl (8.69 g, 125 mmol), the reaction mixture is heated in an 85 °C oil bath (internal temperature: 69 °C) and is stirred for 37 h. The reaction mixture is cooled to ambient temperature and is decanted into sat. brine (150 mL) in a 1-L separatory funnel and is extracted with EtOAc (3 × 200 mL). The combined organic extracts are washed with 3 M NaOH (3 × 100 mL), H2O (100 mL), and brine (100 mL), and then are dried over anhydrous Na2SO4 (20 g), filtered, and concentrated on a rotary evaporator (21 °C 30 mmHg) to afford 5.40 g of the crude diol as a white solid. Purification by recrystallization yields (R,R)-(+)-1,2-diphenyl-1,2-ethanediol as white crystals (4.65 g, 87%).
References
- H. Osajima, H. Fujiwara, K. Okano, H. Tokuyama, T. Fukuyama, Org. Synth. 2009, 86, 130.
PubMed Literature



