Published TCIMAIL newest issue No.199
Maximum quantity allowed is 999

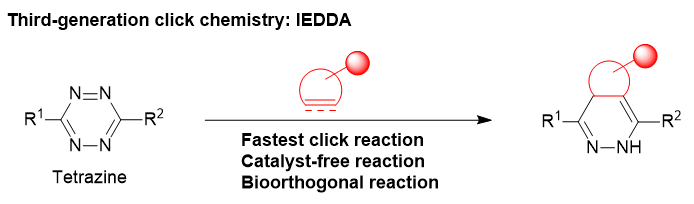
The 1,2,4,5-tetrazines are known to undergo the Strain-Promoted Inverse Electron-Demand Diels-Alder Reaction (SPIEDAC) with strained C-C multiple bonds and are gaining attention as third-generation click reactions. This is a novel method that overcomes the limitations of traditional click reactions, enabling more efficient and specific bonding. Additionally, this reaction meets the criteria for bioorthogonal reactions (fast, selective, biocompatible, catalyst-free) and is being recognized as an innovative technology across various fields such as protein labeling, imaging, and pharmaceutical development.1-4)
Characteristics of Third-Generation Click Reactions
- No need for metal catalysts: Enhanced biocompatibility allows applications in medical and biotechnology fields.
- Fast reactions: Extremely high reaction rates enable efficient experiments and production.
- Selectivity: High selectivity for specific substituents allows desired reactions to proceed even in complex molecular environments.
- Mild reaction conditions: Reactions proceed at room temperature or relatively low temperatures, making handling easy without the need for special conditions.
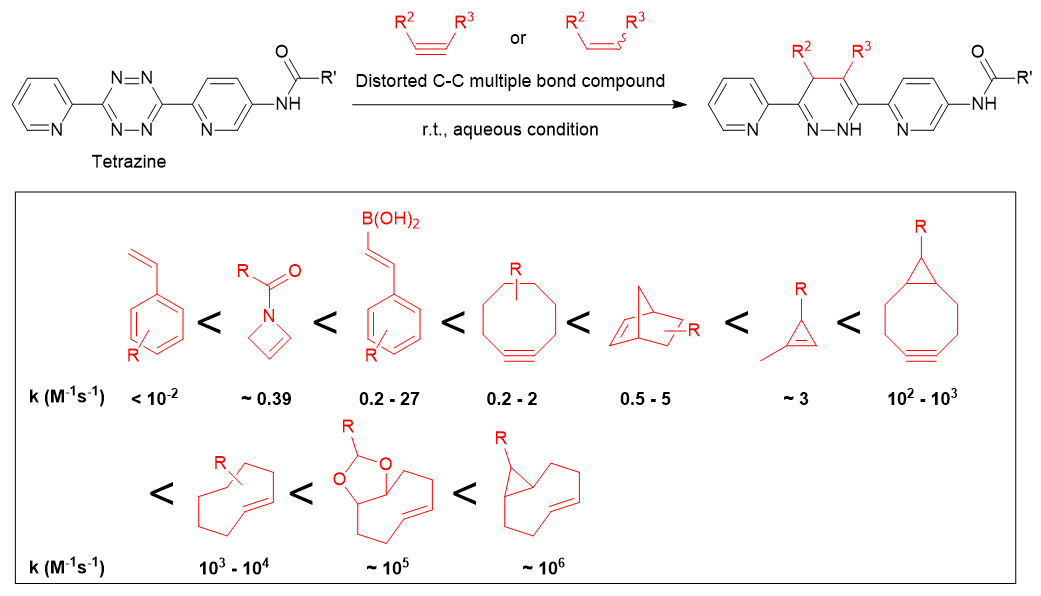
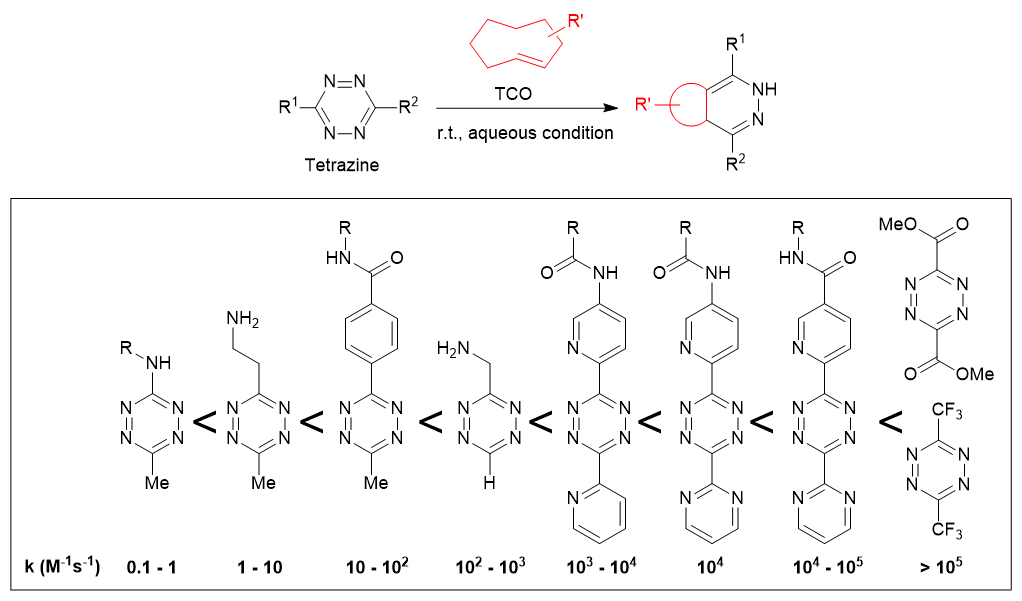
A well-known reaction involving 1,2,4,5-tetrazines is their reaction with TCO (trans-cyclooctene), which occurs at a rate of k = 1 - 106 M-1s-1. This rate is significantly faster compared to the second-generation click reaction (strain-promoted azide-alkyne cycloaddition, SPAAC), which has a reaction rate of approximately k = 10-2 - 10-1 M-1s-1.
It has been suggested that the reaction proceeds much more rapidly when the tetrazine is electron-deficient and has less steric hindrance, and when the reaction partner has a highly strained C-C multiple bond.5)
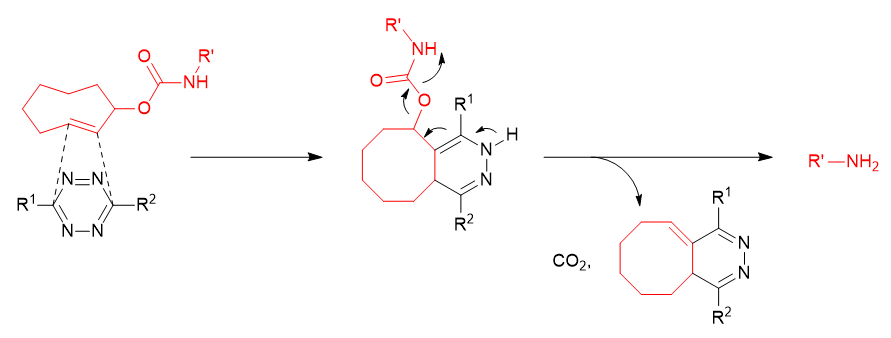
Furthermore, the reaction product of carbamate-type TCO* (2-TCO) derivatives with 1,2,4,5-tetrazines has been reported to undergo a dissociation reaction, releasing an amine. This has raised expectations for its role as a prodrug via a "click-to-release" reaction mechanism.6-9)
Based on these characteristics, we offer a wide range of hetero-bifunctional crosslinkers containing 1,2,4,5-tetrazine. Please use them in conjunction with our TCO derivatives and related products.
Products
Tetrazine-NHS Esters
Tetrazine-Amines
Tetrazine-Azide
Tetrazine-Alkyne
Tetrazine-DBCO
Tetrazine-Biotins
Other Tetrazines
Related Products
TCOs
- T4212
- TCO-NPC equatorial isomer
- T4213
- TCO-NPC axial isomer
- T4218
- TCO*-NPC axial isomer
- T4219
- TCO*-NPC equatorial isomer
- T4234
- TCO-PEG3-amine equatorial isomer
- T4233
- TCO-PEG3-amine axial isomer
- T4258
- TCO*-PEG3-amine equatorial isomer
- T4238
- TCO-PEG3-DBCO axial isomer
- T4239
- TCO-PEG3-DBCO equatorial isomer
- T4260
- TCO*-PEG3-DBCO equatorial isomer
- T4236
- TCO-PEG3-Biotin axial isomer
- T4237
- TCO-PEG3-Biotin equatorial isomer
- T4259
- TCO*-PEG3-Biotin equatorial isomer
- T3949
- TCO-PEG4-NHS
- T4262
- TCO*-PEG3-NHS equatorial isomer
- T3948
- TCO-PEG3-Maleimide
- T4261
- TCO*-PEG3-Maleimide equatorial isomer
References
- 1) Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels−Alder Reactivity
- 2) Fast and Sensitive Pretargeted Labeling of Cancer Cells through a Tetrazine/trans-Cyclooctene Cycloaddition
- 3) Bioorthogonal Turn-On Probes for Imaging Small Molecules inside Living Cells
- 4) Inverse electron demand Diels–Alder reactions in chemical biology
- 5) Tetrazines in Inverse-Electron-Demand Diels–Alder Cycloadditions and Their Use in Biology
- 6) Click to Release: Instantaneous Doxorubicin Elimination upon Tetrazine Ligation
- 7) In situ activation of a doxorubicin prodrug using imaging-capable nanoparticles
- 8) In Vivo Bioorthogonal Chemistry Enables Local Hydrogel and Systemic Pro-Drug To Treat Soft Tissue Sarcoma
- 9) Optimized Tetrazine Derivatives for Rapid Bioorthogonal Decaging in Living Cells





![[4-(6-Methyl-1,2,4,5-tetrazin-3-yl)phenyl]methanamine Hydrochloride](https://www.tcichemicals.com/assets/cms-images/hetero-bifunctional_crosslinkers_containing_tetrazine_M3558.png)










