Maximum quantity allowed is 999
Please select the quantity
CAS RN: 51481-60-8 | Product Number: N1254
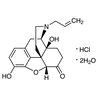
Naloxone Hydrochloride Dihydrate

Purity: >95.0%(HPLC)
Synonyms:
- 17-Allyl-3,14-dihydroxy-5α-4,5-epoxymorphinan-6-one Hydrochloride Dihydrate
Product Documents:
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | N1254 |
Purity / Analysis Method 
|
>95.0%(HPLC) |
| Molecular Formula / Molecular Weight | C__1__9H__2__1NO__4·HCl·2H__2O = 399.87 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive,Hygroscopic,Heat Sensitive |
| CAS RN | 51481-60-8 |
| Reaxys Registry Number | 6260807 |
| MDL Number | MFCD00150901 |
Specifications
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Water | 8.5 to 10.0 % |
| NMR | confirm to structure |
Properties (reference)
| Melting Point | 182 °C |
| Solubility (soluble in) | Methanol |
| Solubility (insoluble in) | Diethyl ether |
GHS
Related Laws:
Transport Information:
| H.S.code* | 2939.19-000 |
Application
Naloxone Hydrochloride: A Competitive Peripheral μ-Opioid Antagonist
Naloxone hydrochloride, a competitive peripheral μ-opioid antagonist, is a synthetic congener of opioid analgesics with little or no μ-opioid agonist properties. It is used clinically for the treatment of opioid dependence and opioid-induced respiratory depression, sedation, and hypotension. (The product is for research purpose only.)
References
- Drugs Five Years Later: Naloxone (a review)
- New and experimental therapeutic roles for naloxone and related opioid antagonists (a review)
- Buprenorphine/naloxone. A review of its use in the treatment of opioid dependence
- Naloxone hydrochloride (A review of the chemistry, synthesis, metabolism, and methods of analysis)
- Development and validation of a HPLC method for the determination of buprenorphine hydrochloride, naloxone hydrochloride, and noroxymorphone in a tablet formulation
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.