Maximum quantity allowed is 999
CAS RN: 33530-51-7 | Product Number: H1766
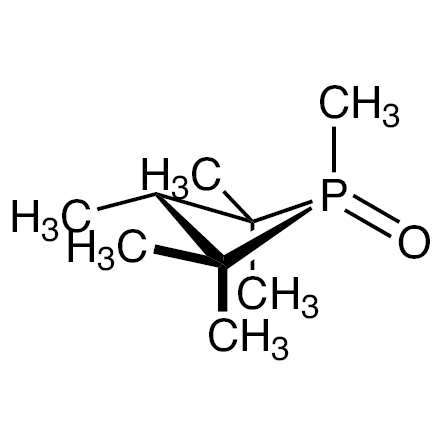
anti-1,2,2,3,4,4-Hexamethylphosphetane 1-Oxide
Purity: >98.0%(GC)
- cis-1,2,2,3,4,4-Hexamethylphosphetane 1-Oxide
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 1G |
$79.00
|
10 | 0 | It can be shipped in about 2 weeks after ordering | |
| 5G |
$250.00
|
7 | 0 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | H1766 |
Purity / Analysis Method 
|
>98.0%(GC) |
| Molecular Formula / Molecular Weight | C__9H__1__9OP = 174.22 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Moisture Sensitive |
Packaging and Container 
|
1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 33530-51-7 |
| Reaxys Registry Number | 1072177 |
| PubChem Substance ID | 468592039 |
| MDL Number | MFCD32857307 |
| Appearance | White to Light yellow powder to crystal |
| Purity(GC) | min. 98.0 % |
| Melting point | 171.0 to 175.0 °C |
| NMR | confirm to structure |
| Melting Point | 174 °C |
| H.S.code* | 2934.99-000 |
References
- PIII/PV=O-Catalyzed Intermolecular N-N Bond Formation: Cross-Selective Reductive Coupling of Nitroarenes and Anilines
- An Improved PIII/PV=O-Catalyzed Reductive C-N Coupling of Nitroaromatics and Boronic Acids by Mechanistic Differentiation of Rate- and Product- Determing Steps

-
Used Chemicals
-
Procedure
-
To a 3-necked 50 mL flask was charged with 1-bromo-2-nitrobenzene (2.006 g, 9.931 mmol, 1.0 equiv.), phenylboronic acid (1.332 g, 10.92 mmol, 1.1 equiv.) and anti-1,2,2,3,4,4-hexamethylphosphetane 1-oxide (0.259 g, 1.49 mmol, 0.15 equiv.). The flask was evacuated on a Schlenk line, and backfilled with argon (3 times). The flask was then charged dry CPME (20 mL), followed by phenylsilane (2.4 mL, 20 mmol, 2.0 equiv.). The mixture was then heated to reflux and stirred for 4 h. The reaction mixture was cooled to room temperature and washed with 1 mol/L NaOH aqueous solution (40 mL). The organic layer was separated and the aqueous layer was extracted with ethyl acetate (20 mL). The combined organic layers were washed with brine (20 mL), dried over Na2SO4 and filtered. The solvent was removed under reduced pressure. The residue was purified by column chromatography (eluent: dichloromethane/ethyl acetate/hexane, 20/1/79) to obtain 1 as a white solid (2.20 g, 89.3%).
-
Experimenter's Comments
-
The reaction mixture was monitored by GC.
CPME was dried over MS4A before use.
-
Analytical Data(Compound 1)
-
1H NMR (400 MHz, CDCl3); δ 7.54 (dd, J = 7.8, 1.4 Hz, 1H), 7.32–7.36 (m, 2H), 7.26–7.28 (m, 1H), 7.16–7.18 (m, 3H), 7.06 (t, J = 7.3 Hz), 6.75 (ddd, J = 7.8, 7.3, 1.4 Hz), 5.79 (brs, 1H).
13C NMR (101 MHz, CDCl3); δ 141.7, 141.5, 133.1, 129.6, 128.2, 122.8, 121.0, 120.4, 115.9, 112.3.
-
Lead Reference
-
- An Improved PIII/PV=O-Catalyzed Reductive C–N Coupling of Nitroaromatics and Boronic Acids by Mechanistic Differentiation of Rate- and Product-Determining Steps
-
Other References
-
- Intermolecular Reductive C–N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV=O Catalysis
[Product Highlights] Organocatalyst for Hydrazine Derivative Synthesis by Cross-Coupling of Nitroarenes and Anilines
[Featured Products] Organophosphorus Catalysts
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts

The requested analytical chart is not available. Sorry for the inconvenience.






![(4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate (4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate](/medias/D5817.jpg?context=bWFzdGVyfHJvb3R8MjU2NzV8aW1hZ2UvanBlZ3xhRGxtTDJnMU5TODVNVEUyT0RJMk1qTTVNREEyTDBRMU9ERTNMbXB3Wnd8YmJkMWY4YTBjMWZkYTI1ZDRhZTVkNzRmZTk3YzM3N2FiZTYzNDUwNGJlNzEzMjJlODIzZDUyMzA0NWNiNThjMQ)
