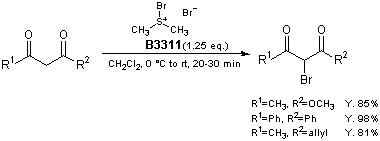Bromodimethylsulfonium bromide (1) is a brominating reagent for various compounds and its utility has been reported.1) For example, β-keto esters and 1,3-diketones are brominated smoothly at 0 °C to room temperature to afford the corresponding α-bromo derivatives selectively in high yields.2) The notable advantages of this protocol are use of reagent 1, which is less hazardous than molecular bromine, and no added base, Lewis acid, or other catalyst.
Moreover, 1 can be used as a catalyst for protection and de-protection of various functional groups.1) Recently, a mild Beckmann Rearrangement using 1 has been reported.3) According to the report, a wide range of ketoximes are converted into the corresponding amides or lactams in high yields.
Maintenance Notice (3:00 AM - 8:30 AM November 1, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Chemistry News October 2025 | [Product Highlights] Tetraphenylethylene Derivatives Used for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)