Maximum quantity allowed is 999
请选择数量
CAS RN: 12092-47-6 | 产品编码: B1045
Chloro(1,5-cyclooctadiene)rhodium(I) Dimer

| 产品编码 | B1045 |
| 分子式/分子量 | C__1__6H__2__4Cl__2Rh__2 = 493.08 |
| 外观与形状(20°C) | 固体 |
储存温度 
|
室温 (15°C以下阴凉干燥处) |
包装和容器 
|
1G-Glass Bottle with Plastic Insert (查看图片) |
| CAS RN | 12092-47-6 |
| Reaxys-RN | 14860700 |
| PubChem物质ID | 87563994 |
| MDL编号 | MFCD00012415 |
技术规格
| Appearance | Light yellow to Brown powder to crystal |
物性(参考值)
GHS
| 象形图 |

|
| 信号词 | Warning |
| 危险性说明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防范说明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
相关法规
运输信息
| HS编码* | 2843.90-000 |
应用
Rh-catalyzed endo- and Enantioselective Hydroacylation of o-Allylbenzaldehydes

Typical Procedure:
In a nitrogen-filled dry box, the appropriate o-allylbenzaldehyde (0.20 mmol, 1.0 eq.), [Rh(COD)Cl]2 (2.5 mg, 0.0050 mmol, 0.025 eq.), (R)-DTBM-SEGPHOS® (11.8 mg, 0.010 mmol, 0.050 eq.), NaBARF (8.9 mg, 0.010 mmol, 0.050 eq.), and anhydrous 1,4-dioxane (1 mL) are added to a 1-dram vial. The vial is sealed with a PFTE/silicone-lined septum cap and removed from the dry box. The reaction mixture is heated to 100 °C and allowed to stir at this temperature until the reaction is judged to be complete by TLC analysis. The mixture is cooled to room temperature, filtered through a pad of silica gel (eluting with EtOAc), and concentrated under reduced pressure. The crude reaction mixture is purified by flash column silica gel chromatography (hexane/EtOAc or hexane/Et2O) to yield the corresponding products.
In a nitrogen-filled dry box, the appropriate o-allylbenzaldehyde (0.20 mmol, 1.0 eq.), [Rh(COD)Cl]2 (2.5 mg, 0.0050 mmol, 0.025 eq.), (R)-DTBM-SEGPHOS® (11.8 mg, 0.010 mmol, 0.050 eq.), NaBARF (8.9 mg, 0.010 mmol, 0.050 eq.), and anhydrous 1,4-dioxane (1 mL) are added to a 1-dram vial. The vial is sealed with a PFTE/silicone-lined septum cap and removed from the dry box. The reaction mixture is heated to 100 °C and allowed to stir at this temperature until the reaction is judged to be complete by TLC analysis. The mixture is cooled to room temperature, filtered through a pad of silica gel (eluting with EtOAc), and concentrated under reduced pressure. The crude reaction mixture is purified by flash column silica gel chromatography (hexane/EtOAc or hexane/Et2O) to yield the corresponding products.
References
应用
Rhodium-Catalyzed Cross-coupling of Organoboron Compounds with Vinyl Acetate

Typical procedure: Under nitrogen atmosphere, a mixture of an arylboron compounds (0.50 mmol), [RhCl(cod)]2 (6.2 mg, 13 µmol), DPPB (11.7 mg, 28 µmol), and K3PO4 (320 mg, 1.5 mmol) is diluted with toluene (2.0 mL). Alkenyl acetate (1.1 or 2.5 mmol) and tert-amyl alcohol (0.55-1.5 mmol) are added into the resulting suspension at room temperature. The mixture is stirred at 100℃ for 24 h and then diluted with hexane (2.0 mL) or EtOAc (2.0 mL). After the filtration through a Celite pad, solvent is removed from the filtrate under reduced pressure. The residue is purified with a flash column chromatography (EtOAc-hexane) to give the desired product.
References
应用
1,2-Addition of Chiral Secondary and Tertiary Alkyl Trifluoroborate
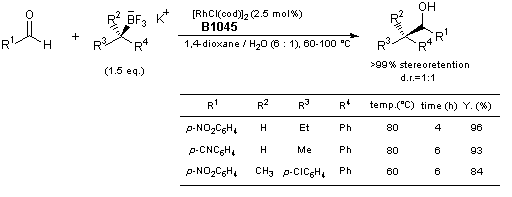
Typical procedure: A dry Schlenk tube flask is charged with the potassium trifluoroborate salt (0.45 mmol), [RhCl(cod)]2 (2.5 mol%, 3.6 mg) and the aldehyde (0.3 mmol). After cycles of vacuum and nitrogen (3 cycles), deoxygenated 1,4-dioxane (1.4 mL) and deoxygenated H2O (240 µL) are added. The reaction mixture is stirred at 60-100°C and the progress of the reaction is checked by TLC. The mixture is cooled to room temperature, saturated NH4Cl solution is added, and then the mixture is extracted with EtOAc. The combined organic layers are dried over MgSO4. Concentration and purification through silica gel column chromatography gives the products.
References
参考文献
文档
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱

请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。






