Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [Product Highlights] Cell Culture-Ready Antibiotic... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
请选择数量
CAS RN: 56390-09-1 | 产品编码: E0840
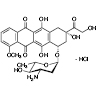
Epirubicin Hydrochloride
技术规格
| Appearance | Light yellow to Yellow to Orange powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Specific rotation | +310 to +340 deg(C=0.05, methanol)(calcd.on anh.substance) |
| Water | max. 8.0 % |
物性(参考值)
| 熔点 | 185 °C |
| 比旋光度 [α]D | 310° (C=0.05,MeOH) |
| 最大吸收波长 | 497 nm (H__2O) |
| 水溶性 | 微溶 |
| 溶解性(微溶于) | 甲醇 |
GHS
| 象形图 |


|
| 信号词 | Danger |
| 危险性说明 | H302 : Harmful if swallowed. H360 : May damage fertility or the unborn child. H340 : May cause genetic defects. H350 : May cause cancer. |
| 防范说明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P270 : Do not eat, drink or smoke when using this product. P202 : Do not handle until all safety precautions have been read and understood. P201 : Obtain special instructions before use. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P308 + P313 : IF exposed or concerned: Get medical advice/ attention. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. P405 : Store locked up. |
相关法规
| RTECS# | QI9295750 |
运输信息
| HS编码* | 2941.90-000 |
应用
Epirubicin Hydrochloride: An Anthracycline Antibiotic Cytotoxic Agent with a Better Toxicity Profile
Epirubicin hydrochloride is the 4’-epimer of the anthracycline antibiotic cytotoxic agent, doxorubicin [D4193]. The precise mechanisms of epirubicin’s cytotoxic and/or antiproliferative properties have not been completely elucidated. However, it has been reported that epirubicin forms a complex with DNA by intercalation of its planar rings between nucleotide base pairs, with consequent inhibition of nucleic acid (DNA and RNA) and protein synthesis. After intercalation between DNA base pairs, epirubicin stabilizes the topoisomerase II-DNA complex, resulting in irreversible DNA strand breakage and cytocidal activity.1-3)
Epirubicin has been shown to have a better toxicity profile than doxorubicin. The formation of glucuronide conjugates, not seen with doxorubicin, is observed with epirubicin. The reason for this is thought to be the inversion of the OH group at the 4' position in epirubicin, which facilitates the reaction of glucuronyl transferase. This facilitates excretion and may contribute to the reduced toxicity of epirubicin compared to doxorubicin.4) In clinical, epirubicin hydrochloride has been used alone or in combination with other cytotoxic chemotherapeutic agents in the treatment of a variety of malignancies. (The product is for research purpose only.)
Epirubicin has been shown to have a better toxicity profile than doxorubicin. The formation of glucuronide conjugates, not seen with doxorubicin, is observed with epirubicin. The reason for this is thought to be the inversion of the OH group at the 4' position in epirubicin, which facilitates the reaction of glucuronyl transferase. This facilitates excretion and may contribute to the reduced toxicity of epirubicin compared to doxorubicin.4) In clinical, epirubicin hydrochloride has been used alone or in combination with other cytotoxic chemotherapeutic agents in the treatment of a variety of malignancies. (The product is for research purpose only.)
References
- 1) Epirubicin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cancer chemotherapy
- 2) Epirubicin: an updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of breast cancer
- 3) Epirubicin: is it like doxorubicin in breast cancer? A clinical review
- 4) Metabolism of 4′-modified analogs of doxorubicin. Unique glucuronidation pathway for 4’-epidoxorubicin
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱

请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。