Product Document Searching Made Easy by 2D Code! | TCI Materials Science News November 2025 | [TCIPracticalExample] Ullmann-type Coupling Reaction at Room Temperature... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
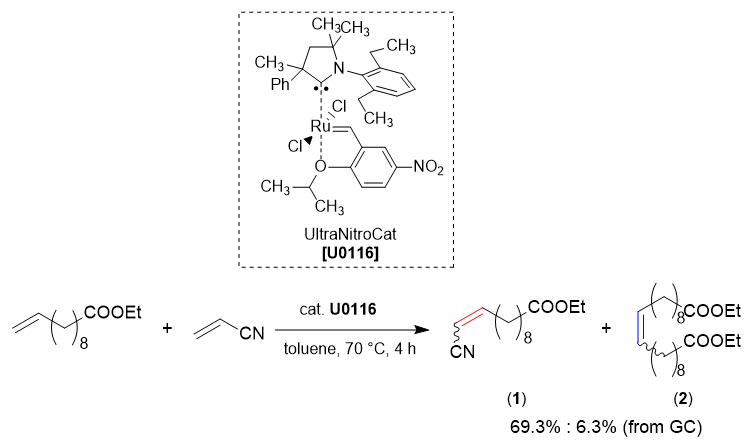
TCI Practical Example: Cross Metathesis Using UltraNitroCat
We are proud to introduce the cross metathesis using UltraNitroCat as a catalyst.
-
Used Chemicals
-
Procedure
-
To a solution of ethyl 10-undecenoate (0.400 mL, 1.7 mmol), acrylonitrile (0.210 mL, 3.2 mmol) in anhydrous toluene (5.8 mL) at 70 °C under nitrogen, a solution of UltraNitroCat (3.4 mg, 0.005 mmol, 0.3 mol%) in toluene (0.5 mL) was dropwised over 1 h. The mixture was stirred at 70 °C under nitrogen for 3 hours. 1,4-Bis(3-isocyanopropyl)piperazine (0.5 mol%, 5.0 mg) was added to the reaction mixture to deactivate the catalyst. The solvent was removed under reduced pressure to obtain a light yellow oil (0.37 g).
The crude oil was analyzed by gas chromatography. The reaction conversion rate of 1 and 2 were 69.3% and 6.3%, respectively. The selectivity ratio of 1 and 2 was 92 : 8.
-
Experimenter's Comments
-
Toluene was dried over molecular sieves 4A and degassed by purging with nitrogen before use.
Preparation of catalyst stock solution: UltraNitroCat (6.9 mg) was dissolved in dry degassed toluene (1.0 mL).
The reaction mixture was analyzed by GC.
-
Analytical Data
-
1: 11-Cyano-10-undecenoic acid ethyl ester
1H NMR (400 MHz, CDCl3); δ 6.47 (m, 1H), 5.30 (m, 1H), 4.12 (m, 2H), 2.41 (m, 2H), 2.03 (m, 2H), 1.61 (m, 2H), 1.43 (m, 2H), 1.22 (m, 11H).
-
2: 10-Eicosenedioic acid diethyl ester
1H NMR (400 MHz, CDCl3); δ 6.71 (m, 2H), 5.36 (m, 2H), 4.12 (m, 4H), 2.21 (m, 4H), 1.96 (m, 4H), 1.61 (m, 4H), 1.43 (m, 4H), 1.22 (m, 22H).
-
Lead Reference
-
- Cyclic Alkyl Amino Ruthenium Complexes—Efficient Catalysts for Macrocyclization and Acrylonitrile Cross Metathesis