Product Document Searching Made Easy by 2D Code! | TCI Materials Science News November 2025 | [TCIPracticalExample] Ullmann-type Coupling Reaction at Room Temperature... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 22457-89-2 | Product Number: B4711
Benfotiamine
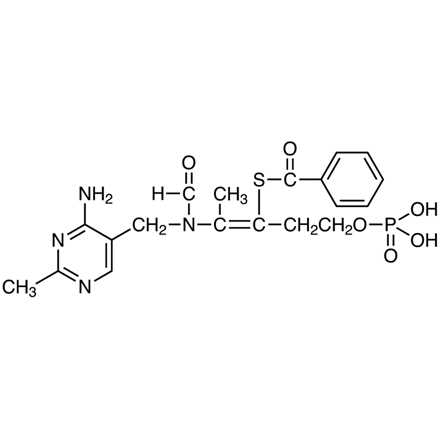
Purity: >98.0%(T)(HPLC)
Synonyms:
- S-Benzoylthiamine O-Monophosphate
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 5G |
NT$2,688
|
22 | ≥40 | Contact Us | |
| 25G |
NT$8,106
|
12 | 5 | Contact Us |
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | B4711 |
Purity / Analysis Method 
|
>98.0%(T)(HPLC) |
| Molecular Formula / Molecular Weight | C__1__9H__2__3N__4O__6PS = 466.45 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Refrigerated (0-10°C) |
| Condition to Avoid | Heat Sensitive |
| CAS RN | 22457-89-2 |
| Reaxys Registry Number | 771326 |
| PubChem Substance ID | 253662524 |
| Merck Index (14) | 1041 |
| MDL Number | MFCD00057343 |
Specifications
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(Neutralization titration) | min. 98.0 % |
Properties (reference)
| Melting Point | 192 °C(dec.) |
GHS
Related Laws:
| RTECS# | DH6910000 |
Transport Information:
| H.S.code* | 2933.59-000 |
Application
Paraptosis research
Reference
- Paraptosis Cell Death Induction by the Thiamine Analog Benfotiamine in Leukemia Cells
- Pharmacokinetics of thiamine derivatives, especially of benfotiamine (a review)
- D. Loew, Int. J. Clin. Pharmacol. Ther. 1996, 34, 47.
- Efficacy of benfotiamine versus thiamine on function and glycation products of peripheral nerves in diabetic rats
- Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy
- Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes
- The multifaceted therapeutic potential of benfotiamine (a review)
Application
Benfotiamine: S-Acyl Derivative of Thiamine with High Bioavailability
S-benzoylthiamine O-monophoshate (benfotiamine) is a synthetic S-acyl derivative of thiamine (vitamin B1). Benfotiamine is absorbed in vivo much more than water soluble thiamine hydrochloride [T0181]. Benfotiamine increases the levels of intracellular thiamine diphosphate, a cofactor necessary for the activation transketolase, resulting in the reduction of tissue level of advanced glycation end products (AGEs) which can be a factor in the development or worsening of many degenerative diseases, such as diabetes, atherosclerosis, chronic renal failure and Alzheimer's disease.
References
PubMed Literature
Product Articles
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.



