Maximum quantity allowed is 999
Please select the quantity
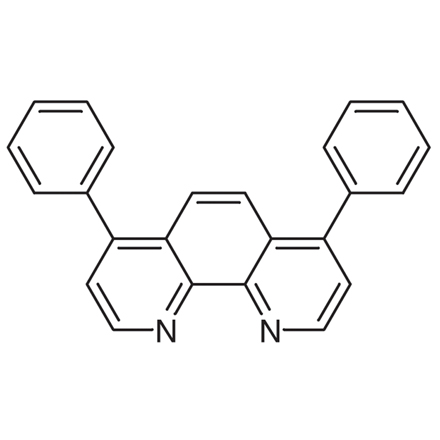
CAS RN: 1662-01-7 | Product Number: D0905
Bathophenanthroline
Purity: >99.0%(T)
Synonyms:
- 4,7-Diphenyl-1,10-phenanthroline
- Bphen
Product Documents:
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | D0905 |
Purity / Analysis Method 
|
>99.0%(T) |
| Molecular Formula / Molecular Weight | C__2__4H__1__6N__2 = 332.41 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
Packaging and Container 
|
1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 1662-01-7 |
| Reaxys Registry Number | 261048 |
| PubChem Substance ID | 87567505 |
| SDBS (AIST Spectral DB) | 3200 |
| MDL Number | MFCD00004976 |
Specifications
| Appearance | White to Orange to Green powder to crystal |
| Purity(Nonaqueous Titration) | min. 99.0 % |
| Purity(HPLC) | min. 98.0 area% |
| Sensitiveness | Abs min.0.36(near 533nm) in the presence of Fe(1 ppm) |
Properties (reference)
| Melting Point | 221 °C |
| Solubility in water | Slightly soluble |
| Solubility (soluble in) | Benzene, Acetone, Ethanol |
GHS
Related Laws:
| RTECS# | SF8427000 |
Transport Information:
| H.S.code* | 2933.99-000 |
Application
Arylation via C-H Activation using an Organocatalyst
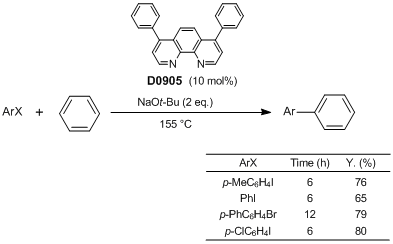
Typical Procedure: A mixture of bathophenanthroline (7.6 mg, 23 µmol), sodium tert-butoxide (43.3 mg, 0.451 mmol), 4-iodotoluene (48.6 mg, 0.223 mmol) and benzene (2.4 mL, 27 mmol) in a 35 mL oven-dried pressure-resistant tube is stirred at 155 °C for 6 h. After cooling, the reaction mixture is quenched with a 1 N HCl aqueous solution (4 mL) and extracted with Et2O (10 mL × 3). The combined organic layer is dried over MgSO4, filtered, and concentrated in vacuo. Purification with PTLC (silica gel, hexane : EtOAc = 20 : 1) gives the product (28.4 mg, Y. 76%).
References
Application
Iron-catalyzed direct arylation of benzenes

Typical procedure: To a vial is added Fe(OAc)2 (4.3 mg), bathophenanthroline (16.6 mg), and KOt-Bu (112 mg). To the vial is then added iodobenzene (102 mg) and benzene (3.9 g). The reaction was stirred vigorously at room temperature for 20 min and then at 80 °C for 20 h. Following cooling, 2 mL of CH2Cl2/hexanes (1:1) is added, and the solution is filtered through silica pad. The pad then rinsed with 15 mL of CH2Cl2/hexanes (1:1). The combined solution is concentrated and the crude mixture is purified by column chromatography (eluent: hexanes) to give biphenyl (68.6 mg, 89 %) as a white solid.
References
PubMed Literature
Documents
Product Articles
[Product Highlights] Copper/photoredox-catalyzed Decarboxylative sp3 C–N Coupling Reaction of N-Heteroaromatics[Research Articles] Arylation via C-H Activation using an Organocatalyst
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





![(4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate (4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate](/medias/D5817.jpg?context=bWFzdGVyfHJvb3R8MjU2NzV8aW1hZ2UvanBlZ3xhRGxtTDJnMU5TODVNVEUyT0RJMk1qTTVNREEyTDBRMU9ERTNMbXB3Wnd8YmJkMWY4YTBjMWZkYTI1ZDRhZTVkNzRmZTk3YzM3N2FiZTYzNDUwNGJlNzEzMjJlODIzZDUyMzA0NWNiNThjMQ)


