Maximum quantity allowed is 999
Please select the quantity
CAS RN: 51481-60-8 | Product Number: N1254
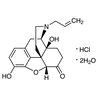
Naloxone Hydrochloride Dihydrate

Purity: >95.0%(HPLC)
Synonyms:
- 17-Allyl-3,14-dihydroxy-5α-4,5-epoxymorphinan-6-one Hydrochloride Dihydrate
Product Documents:
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | N1254 |
Purity / Analysis Method 
|
>95.0%(HPLC) |
| Molecular Formula / Molecular Weight | C__1__9H__2__1NO__4·HCl·2H__2O = 399.87 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive,Hygroscopic,Heat Sensitive |
| CAS RN | 51481-60-8 |
| Reaxys Registry Number | 6260807 |
| MDL Number | MFCD00150901 |
Specifications
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Water | 8.5 to 10.0 % |
| NMR | confirm to structure |
Properties (reference)
| Melting Point | 182 °C |
| Solubility (soluble in) | Methanol |
| Solubility (insoluble in) | Diethyl ether |
GHS
Related Laws:
Transport Information:
| H.S.code* | 2939.19-000 |
Application
Naloxone Hydrochloride: A Competitive Peripheral μ-Opioid Antagonist
Naloxone hydrochloride, a competitive peripheral μ-opioid antagonist, is a synthetic congener of opioid analgesics with little or no μ-opioid agonist properties. It is used clinically for the treatment of opioid dependence and opioid-induced respiratory depression, sedation, and hypotension. (The product is for research purpose only.)
References
- Drugs Five Years Later: Naloxone (a review)
- New and experimental therapeutic roles for naloxone and related opioid antagonists (a review)
- Buprenorphine/naloxone. A review of its use in the treatment of opioid dependence
- Naloxone hydrochloride (A review of the chemistry, synthesis, metabolism, and methods of analysis)
- Development and validation of a HPLC method for the determination of buprenorphine hydrochloride, naloxone hydrochloride, and noroxymorphone in a tablet formulation
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.