Maximum quantity allowed is 999
Please select the quantity
CAS RN: 888504-28-7 | Product Number: P2220
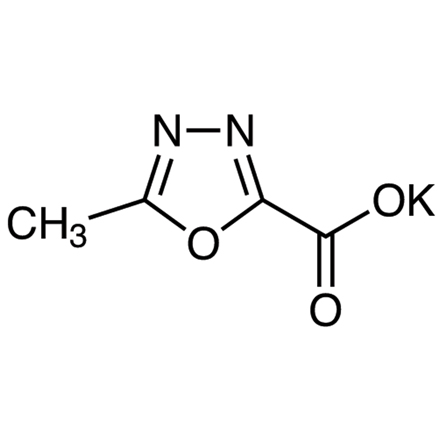
Potassium 5-Methyl-1,3,4-oxadiazole-2-carboxylate
Purity: >98.0%(T)(HPLC)
Synonyms:
- 5-Methyl-1,3,4-oxadiazole-2-carboxylic Acid Potassium Salt
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 200MG |
NT$1,218
|
11 | 1 | Contact Us | |
| 1G |
NT$4,746
|
15 | 0 | Contact Us |
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | P2220 |
Purity / Analysis Method 
|
>98.0%(T)(HPLC) |
| Molecular Formula / Molecular Weight | C__4H__3KN__2O__3 = 166.18 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 888504-28-7 |
| Reaxys Registry Number | 14557884 |
| PubChem Substance ID | 354334121 |
| MDL Number | MFCD08060085 |
Specifications
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(Nonaqueous Titration) | min. 98.0 % |
| NMR | confirm to structure |
Properties (reference)
GHS
Related Laws:
Transport Information:
| H.S.code* | 2934.99-000 |
Application
A Synthetic Intermediate of Raltegravir
This product is used for the synthesis of raltegravir which is an HIV integrase inhibitor used for the treatment of human immunodeficiency virus (HIV) infection.
References
- Discovery of Raltegravir, a Potent, Selective Orally Bioavailable HIV-Integrase Inhibitor for the Treatment of HIV-AIDS Infection
- Development of a Second-Generation, Highly Efficient Manufacturing Route for the HIV Integrase Inhibitor Raltegravir Potassium
- Identification, Synthesis, and Strategy For Minimization of Potential Impurities Observed In Raltegravir Potassium Drug Substance
PubMed Literature
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.



