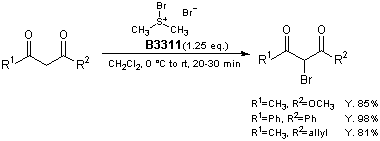Bromodimethylsulfonium bromide (1) is a brominating reagent for various compounds and its utility has been reported.1) For example, β-keto esters and 1,3-diketones are brominated smoothly at 0 °C to room temperature to afford the corresponding α-bromo derivatives selectively in high yields.2) The notable advantages of this protocol are use of reagent 1, which is less hazardous than molecular bromine, and no added base, Lewis acid, or other catalyst.
Moreover, 1 can be used as a catalyst for protection and de-protection of various functional groups.1) Recently, a mild Beckmann Rearrangement using 1 has been reported.3) According to the report, a wide range of ketoximes are converted into the corresponding amides or lactams in high yields.
Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [TCIPracticalExample] Suzuki-Miyaura Coupling Using Encapsulated... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)