Useful Linker
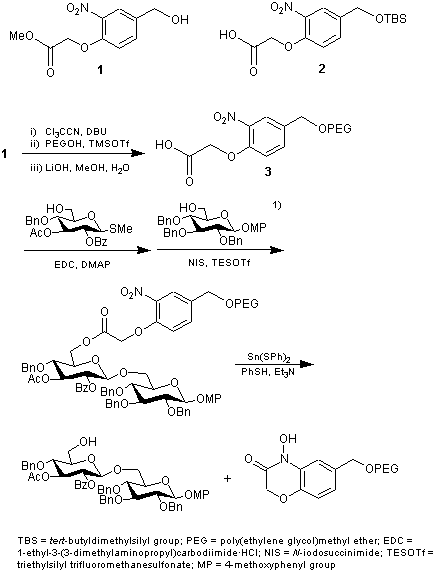
Linkers 1 and 2 developed by Manabe, et al. can be utilized for oligosaccharide synthesis. Polymer 3 derived from 1 has a strong electron-withdrawing nitro group, and is stable under acidic condition. Thus, glycosylation under acidic conditions can be carried out. After reaction, due to intramolecular cyclization occured by reduction of nitro group, cleavage of oligosaccharide from the polymer can be readily accomplished.1) These linkers are not only useful for oligosaccharide synthesis, but also they are expected to be utilized for many other reactions with combination of Lewis acid.
References
- 1)Linker for polymer support oligosaccharide synthesis
- 2)A new phenoxyacetate-based linker system for the solid-phase synthesis of oligosaccharides
- 3)Review
- S. Manabe, Yakugaku Zassi 2002, 122, 295.
- S. Manabe, Kagaku To Kogyo (Tokyo) 2002, 55, 145.
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.