Sign up for free shipping on all website orders, no minimum required, and get exclusive coupons!
Users can now ask questions to the AI chatbot by clicking the chatbot icon in the bottom-right corner of the website.
Maximum quantity allowed is 999
| Size | Unit Price | Philadelphia, PA | Portland, OR | Japan* | Quantity |
Shipping Information

|
|---|---|---|---|---|---|---|
| 1G |
$65.00
|
Contact Us | Contact Us | 20 |
|
|
| 10G |
$200.00
|
Ships within 5 weeks after ordering | Ships within 3 weeks after ordering | ≥40 |
|
* Items in stock locally typically ship the same day of ordering. Items from Japan stock are able to ship from a US warehouse within 2 weeks after ordering. For additional estimated shipment times, please refer to the Shipping Simulation tool. Note: Excludes regulated items and items that ship on ice.
* To send your quote request for bulk quantities, please click on the "Request Quote" button. Please note that we cannot offer bulk quantities for some products.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
| Product Number | B6316 |
Purity / Analysis Method 
|
>95.0%(T)(HPLC) |
| Molecular Formula / Molecular Weight | C__1__4H__1__9NO__6 = 297.31 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive |
| CAS RN | 19350-66-4 |
| Reaxys Registry Number | 489397 |
| PubChem Substance ID | 468591034 |
| MDL Number | MFCD00417306 |
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Purity(Neutralization titration) | min. 95.0 % |
| NMR | confirm to structure |
| Melting Point | 220 °C |
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P302 + P352 : IF ON SKIN: Wash with plenty of soap and water. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. P362 : Take off contaminated clothing and wash before reuse. |
| HS Number | 2933.39.9200 |
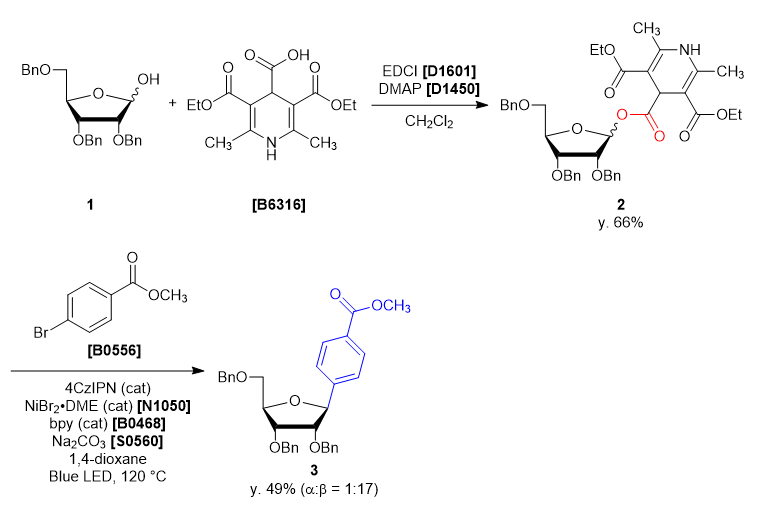
A four-neck round bottom flask was charged with 2,3,5-tri-O-benzyl-α/β-D-ribofuranose (1) (2.165 g, 5.15 mmol, 1 eq) and dichloromethane (26 mL). The solution was cooled under 5 ˚C, then DMAP (0.063 g, 0.51 mmol, 0.1 eq), EDCI (1.38 g, 7.21 mmol, 1.4 eq), 3,5-bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic acid (1.684 g, 5.66 mmol, 1.1 eq) was added. The reaction mixture was allowed to warm to ambient temperature and stirred for 46 h. After the reaction, the reaction mixture was quenched with ion-exchanged water (35 mL). The solution was transferred into a separatory funnel, and the aqueous layer was extracted with dichloromethane (10 mL, twice). The combined organic layers were washed with brine, dried over sodium sulfate (10 g) for about 30 minutes and then filtered. The solvent was removed in vacuo, giving crude as a blown oil (3.91 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 1:1, Rf = 0.45) to give compound 2 as a light yellow oil (2.99 g, y. 66%).
30 mL sealed vessel was charged with 4CzIPN (0.0158 g, 0.020 mmol, 0.1 eq), methyl 4-bromobenzoate (0.0858 g, 0.399 mmol, 2.0 eq), sodium carbonate (0.0443 g, 0.418 mmol, 2.1 eq), 2,2’-bipyridine (0.0086 g, 0.055 mmol, 0.28 eq) at rt under N2. A solution of NiBr2・DME (0.012 g, 0.040 mmol, 0.20 eq) in 1,4-dioxane (5 mL) and the solution of 1 (0.14 g, 0.20 mmol, 1.0 eq) in 1,4-dioxane (5 mL) were added to the sealed vessel. The mixture was placed in a preheated oil bath whose temperature was set to 120 ˚C, and placed at a distance of 2-3 cm from Blue LED lamp. After irradiation for 46 h, the reaction mixture was cooled to room temperature and filtered through Celite pad (1 cm). The solvent was removed in vacuo and the crude was given as a yellow oil (0.23 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 10:2, Rf = 0.30) to give compound 3 as a colorless oil (0.053 g, y. 49%).
Compound 3
1H NMR (270 MHz, CDCl3); δ 7.96 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.40-7.30 (m, 11H), 7.26-7.23 (m, 2H), 7.18-7.15 (m, 2H), 5.05 (d, J = 6.9 Hz, 1H), 4.71-4.44 (m, 6H), 4.39-4.34 (m, 1H), 4.01 (dd, J = 5.3, 3.3 Hz, 1H), 3.92 (s, 3H), 3.77 (dd, J = 6.9, 5.3 Hz, 1H), 3.65 (qd, J = 10.6, 3.9 Hz, 2H).
The requested SDS is not available.
Please Contact Us for more information.
A sample C of A for this product is not available at this time.

The requested analytical chart is not available. Sorry for the inconvenience.
