网站维护通知 (2025年12月13日11:00~16:30,12月14日6:30~8:40):由于系统维护,网站暂时不可用,敬请谅解!
联系方式:021-67121386 / Sales-CN@TCIchemicals.com
Maximum quantity allowed is 999
请选择数量
聚合物/大分子半导体砌块
Polyacetylenes exhibit semiconducting-insulating properties by a lack of free electrons (carriers), although there are conducting pathways through π-conjugations. Shirakawa, MacDiarmid and Heeger et al. in 1977 observed that polyacetylenes show high electrical conduction comparable with a metal when the insulating polyacetylenes obtain carriers by a bromine doping.1) After their observation, studies of conducting polymers were dramatically enhanced, and some of the representative polymers were practical for electronic equipment.
In 1990s, an organic light-emitting diode (OLED) of polyphenylene vinylene (PPV) was reported and then semiconducting polymers attracted us in this research area.2) The PPV is one of the light-emitting polymers (LEP) and the functionality is due to the semiconducting property of a π-conjugated polymer without a chemical doping. A chemical modification of a π-conjugated polymer can create various colored emissions. For instance, polyfluorenes (PFO),3) PPVs4) and regiorandom poly(3-octylthiophene) (P3OT)5) exhibit blue, green and red emissions, respectively.
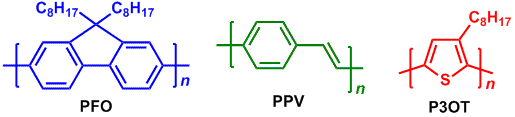
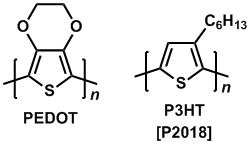
Recent research on organic electronics developed sulfur-containing polymers such as polythiophene. Poly(3,4-ethylenedioxythiophene) (PEDOT) is useful for a capacitor, organic transistor, hole transport material for an OLED device, and secondary battery as well as for an actuator, sensor and thermoelectric conversion element.6,7) PEDOT/PSS that is mixture of PEDOT and polystyrene sulfonate (PSS), is widely used as a hole transport material for organic photovoltaics (OPV).8) Poly(3-hexyl)thiophene (P3HT) can be blended with fullerene derivatives (eg. PCBM) to form an efficient bulk heterojunction. The polymer for the OPV device works as a p-type semiconductor with light absorption.
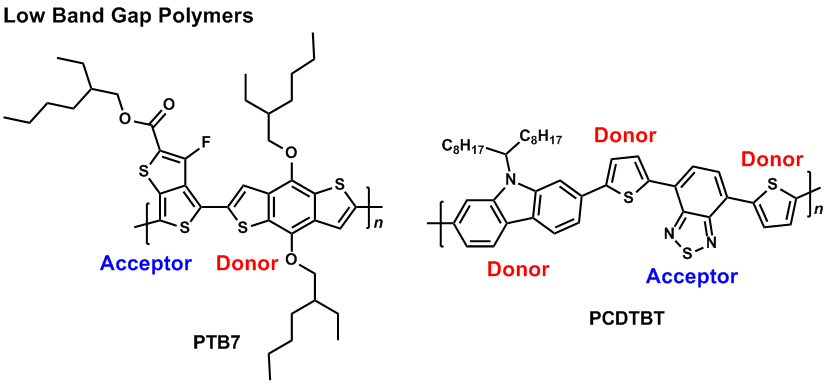
On the other hand, some thiophene-based polymers exhibit a high energy level of the highest occupied molecular orbital (HOMO), because they are electron-rich. One problem of the thiophene-based polymers is their sensitivity for electrochemical oxidation. In order to solve the problem, one can combine an electron-rich monomer (donor) and an electron-deficient monomer (acceptor) to decrease the HOMO energy level and then stable donor-acceptor (DA type) polymers can be produced.10-12) The DA type polymer usually shows a low band gap state which can absorb light of long wavelength. Furthermore, several DA type polymers are ambipolar enabling both p- and n-dopes.13,14)
Topics and Information on Building Blocks for Organic Semiconductor Synthesis
Related Product Category Pages
References
- 1) H. Shirakawa, E. J. Louis, A. G. MacDiarmid, C. K. Chiang, A. J. Heeger, J. Chem. Soc., Chem. Comm. 1977, 578.

- 2) J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns, A. B. Holmes, Nature 1990, 347, 539.

- 3) A. W. Grice, D. D. C. Bradley, M. T. Bernius, M. Inbasekaran, W. W. Wu, E. P. Woo, Appl. Phys. Lett. 1998, 73, 629.

- 4) Review: A. B. Holmes, D. D. C. Bradley, A. R. Brown, P. L. Burn, J. H. Burroughes, R. H. Friend, N. C. Greenham, R. W. Gymer, D. A. Halliday, R. W. Jackson, A. Kraft, J. H. F. Martens, K. Pichler, I. D. W. Samuel, Synth. Met. 1993, 57, 4031.

- 5) D. Braun, G. Gustafsson, D. McBranch, A. J. Heeger, J. Appl. Phys. 1992, 72, 564.

- 6) Review: L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik, J. R. Reynolds, Adv. Mater. 2000, 12, 481.

- 7) A. Elschner, S. Kirchmeyer, W. Lovenich, U. Merker, K. Reuter, in PEDOT: Principles and Applications of an Intrinsically Conductive Polymer, CRC Press, 2010.
- 8) Review: C. Winder, N. S. Sariciftci, J. Mater. Chem. 2004, 14, 1077.

- 9) Review: M. T. Dang, L. Hirsch, G. Wantz, Adv. Mater. 2011, 23, 3597.

- 10) Y. Liang, Z. Xu, J. Xia, S.-T. Tsai, Y. Wu, G. Li, C. Ray, L. Yu, Adv. Mater. 2010, 22, E135.

- 11) N. Blouin, A. Michaud, M. Leclerc, Adv. Mater. 2007, 19, 2295.

- 12) P. M. Beaujuge, W. Pisula, H. N. Tsao, S. Ellinger, K. Müllen, J. R. Reynolds, J. Am. Chem. Soc. 2009, 131, 7514.

- 13) H. Ito, T. Iwata, S. Watanabe, S. Kuroda, Appl. Phys. Express 2013, 6, 051601.

- 14) S. Cho, J. H. Seo, G.-H. Kim, J. Y. Kim, H. Y. Woo, J. Mater. Chem. 2012, 22, 21238.


