Porphyrin has a cyclic structure with four condensed pyrroles. The corresponding porphyrin metal complexes are important in-vivo, because they are included in chlorophyll functioning photo absorption and photo electron transfer for photosynthesis, and also included in heme (hemoglobin) transporting oxygen in blood. In addition, porphyrinato metal complexes are useful for photoelectron functional materials, metal complex catalysts and molecular electrical conductors. Chemical modifications of substituted groups, metal centers, and axial ligands on the porphyrinato metal complexes show various functionalities. Normal porphyrin complexes have sharp absorptions, the so-called Soret band, around 400-500 nm, and the Q band around 500-700 nm that is a relatively weak absorption. Molar absorption coefficients of the Soret band are up to order of 106 M/cm. The chlorophyll and porphyrinato zinc complexes hardly show energy relaxation of absorbed light, but easily show photoelectron transfer. Therefore, studies on porphyrinato metal complexes are performed for artificial photosynthesis (eg. reduction of carbon dioxide) and solar cell materials.
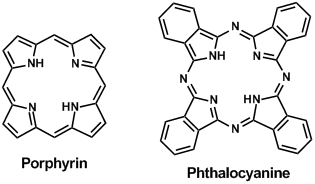
Phthalocyanine compounds do not exist in nature, although they have an analogue structure to porphyrin compounds. The phthalocyanato metal complexes are artificial dyes for painting of railway trains, and for organic photoconductors of electrophotography. There are applications of organic semiconductors as organic transistors and hole injection materials for organic light-emitting diodes (OLED). The phthalocyanato metal complexes show a more intense Q band absorption than that of the Soret band. Their absorption wavelengths are shifted to longer wavelengths than those of porphyrin compounds. Absorption wavelength is further shifted to the near infrared area by modification of the central metal and expansion of the π-conjugates. Introduction of an alkyl group provides soluble phthalocyanine and porphyrin compounds, although they are poorly soluble due to the large π-conjugated cycle.














