盲盒好礼,精彩继续 | 轻松扫码查看产品文档 | TCIMAIL No.198 已上新 | TCI试剂——品质可靠,值得信赖
订购方法?联系方式:021-67121386 / Sales-CN@TCIchemicals.com
Maximum quantity allowed is 999
请选择数量
CAS RN: 12112-67-3 | 产品编码: C1807
Chloro(1,5-cyclooctadiene)iridium(I) Dimer

* 点击“查询”可查看预计发货日期,仅供参考。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
| 产品编码 | C1807 |
| 纯度/分析方法 | >93.0%(T) |
| 分子式/分子量 | C__1__6H__2__4Cl__2Ir__2 = 671.70 |
| 外观与形状(20°C) | 固体 |
| 储存温度 | 冷藏 (0-10°C) |
| 储存在惰性气体下 | 存放于惰性气体之中 |
| 应避免的情况 | 空气,加热 |
| 包装和容器 | 1G-带有塑料内管的玻璃瓶 (查看图片), 250MG-带有塑料内管的玻璃瓶 (查看图片) |
| CAS RN | 12112-67-3 |
| Reaxys-RN | 14372408 |
| PubChem物质ID | 87560730 |
| MDL编号 | MFCD00012414 |
技术规格
| Appearance | Orange to Amber to Dark red powder to crystal |
| Purity(Argentometric Titration) | min. 93.0 % |
物性(参考值)
| 熔点 | 205 °C(dec.) |
| 水溶性 | 不溶 |
GHS
| 象形图 |

|
| 信号词 | 警告 |
| 危险性说明 | H315 : 造成皮肤刺激。 H319 : 造成严重眼刺激。 |
| 防范说明 | P264 : 作业后彻底清洗皮肤。 P280 : 戴防护手套/戴防护眼罩/戴防护面具。 P302 + P352 : 如皮肤沾染:用水充分清洗。 P337 + P313 : 如仍觉眼刺激:求医/就诊。 P305 + P351 + P338 : 如进入眼睛:用水小心冲洗几分钟。如戴隐形眼镜并可方便地取出,取出隐形眼镜。继续冲洗。 P362+P364 : 脱掉沾污的衣服,清洗后方可重新使用。 P332 + P313 : 如发生皮肤刺激:求医/就诊。 |
相关法规
| 新化学物质备案回执号 | B1A232216304 |
运输信息
| 监管条件代码(*) |
应用
Iridium / Axially Chiral Phosphoramidite Complex Catalyzed Allylic Substitution of Allyl Carbonate Derivatives

In a nitrogen-filled atmosphere, [Ir(cod)Cl]2 (2.7 mg, 0.0040 mmol), the ligand (4.3 mg, 0.0080 mmol), KF (12 mg, 0.20 mmol), 18-crown-6 (53 mg, 0.20 mmol) and a Teflon-coated magnetic stirring bar are added to a 1-dram vial. Then, anhydrous THF (0.40 mL) is added. The mixture is stirred for 5 min. at ambient temperature before allyl carbonate derivative (0.20 mmol) and the silyl enolate derivative (0.40 mmol, 2.0 equiv) are added. The vial is sealed with a cap and stirred at 50 °C for 12 h. Then, water (1 mL) is added to the reaction mixture followed by 1 N HCl (0.5 mL). The resulting mixture is stirred vigorously for 3 h at room temperature. Brine (1 mL) and Et2O (1 mL) are added; the organic layer is separated, and the aqueous layer is extracted with Et2O (3 x 3 mL). The combined organic extracts are dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product is performed by flash column chromatography (gradient elution: hexane:Et2O = 50:1 to 5:1) to provide the product.
References
- Iridium-Catalyzed Enantioselective Allylic Substitution of Unstabilized Enolates Derived from α,β-Unsaturated Ketones
应用
C-H Borylation of Arenes

Typical Procedure:
A 25 mL-flask assembled a magnetic stirring bar, a septum inlet, and a condenser is charged with chloro(1,5-cyclooctadiene)iridium(I) dimer (10.1 mg, 0.015 mmol), 2,2’-bipyridyl (4.7 mg, 0.03 mmol), and bis-(pinacolato)diboron (254.0 mg, 1.0 mmol) and then flushed with nitrogen. An arene (60 mmol) is added, and the mixture is stirred at 80 °C for 16 h. The reaction mixture is analyzed by GC and GC mass spectroscopy. The product is extracted with benzene, washed with brine, and dried over MgSO4. Kugelrohr distillation gives analytically pure samples.
A 25 mL-flask assembled a magnetic stirring bar, a septum inlet, and a condenser is charged with chloro(1,5-cyclooctadiene)iridium(I) dimer (10.1 mg, 0.015 mmol), 2,2’-bipyridyl (4.7 mg, 0.03 mmol), and bis-(pinacolato)diboron (254.0 mg, 1.0 mmol) and then flushed with nitrogen. An arene (60 mmol) is added, and the mixture is stirred at 80 °C for 16 h. The reaction mixture is analyzed by GC and GC mass spectroscopy. The product is extracted with benzene, washed with brine, and dried over MgSO4. Kugelrohr distillation gives analytically pure samples.
References
- Mild iridium-catalyzed borylation of arenes. High turnover numbers, room temperature reactions, and isolation of a potential intermediate
应用
Iridium-catalyzed reaction of alcohols with enones affording 1,3-diketones

Typical procedure (R=H):
A mixture of [IrCl(cod)]2 (34 mg, 0.05 mmol), P(n-Oct)3 (74 mg, 0.2 mmol), i-PrOH (180 mg, 3 mmol), Na2SO3 (50 mg, 0.4 mmol), benzyl alcohol (108 mg, 1 mmol) and 2-cyclohexen-1-one (481 mg, 5 mmol) in p-xylene (1 mL) is stirred at 120 °C for 15 h under an argon atmosphere in a pressure tube. The product is purified by column chromatography on 230-400 mesh silica gel (eluent: hexane/ethyl acetate = 40/1) to give the desired 1,3-diketone (142 mg, 70% yield).
A mixture of [IrCl(cod)]2 (34 mg, 0.05 mmol), P(n-Oct)3 (74 mg, 0.2 mmol), i-PrOH (180 mg, 3 mmol), Na2SO3 (50 mg, 0.4 mmol), benzyl alcohol (108 mg, 1 mmol) and 2-cyclohexen-1-one (481 mg, 5 mmol) in p-xylene (1 mL) is stirred at 120 °C for 15 h under an argon atmosphere in a pressure tube. The product is purified by column chromatography on 230-400 mesh silica gel (eluent: hexane/ethyl acetate = 40/1) to give the desired 1,3-diketone (142 mg, 70% yield).
References
应用
Iridium-catalyzed alpha-alkylation of tert-butyl acetate

Typical procedure:
A mixture of [IrCl(cod)]2 (34 mg), PPh3 (39 mg), tert-BuOK (224 mg), n-butanol (74 mg) and tert-butyl acetate (449 mg) in tert-BuOH (1 mL) is stirred at 100 °C for 15 h under Ar. The product (tert-butyl hexanoate) is obtained in 74% yield (estimated using GC), and is isolated by column chromatography on 230-400 mesh silica-gel (eluent: hexane/THF = 50/1) in 62 % yield (107 mg).
A mixture of [IrCl(cod)]2 (34 mg), PPh3 (39 mg), tert-BuOK (224 mg), n-butanol (74 mg) and tert-butyl acetate (449 mg) in tert-BuOH (1 mL) is stirred at 100 °C for 15 h under Ar. The product (tert-butyl hexanoate) is obtained in 74% yield (estimated using GC), and is isolated by column chromatography on 230-400 mesh silica-gel (eluent: hexane/THF = 50/1) in 62 % yield (107 mg).
References
应用
Selective Synthesis of Diketones and omega-Hydroxy Ketones by [IrCl(cod)]2/PPh3/KOH System
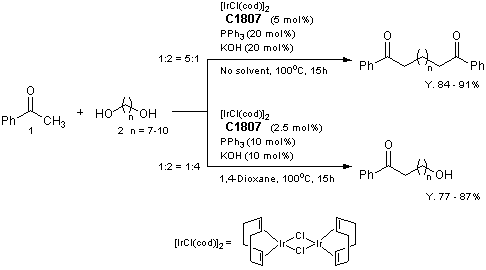
Reference
参考文献
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。






