Maintenance Notice (3:00 AM - 8:30 AM November 1, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Chemistry News October 2025 | [Product Highlights] Tetraphenylethylene Derivatives Used for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
Glycosyl Donor
No.149(December 2012)
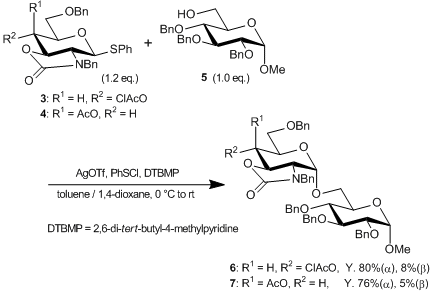
Phenyl N-benzyl-2-amino-4,6-O-benzylidene-2-N,3-O-carbonyl-2-deoxy-1-thio-β-D-glucopyranoside (1) is a glycosyl donor developed by Mababe et al., which is used as a building block for 1,2-cis glycosidic bond formation of an amino sugar. For example, reductive benzylidene acetal ring opening of 1 gives 2. Subsequently, 2 is converted either to glucosamine derivative 3 via chloroacetylation or to galactosamine derivative 4 via stereoinversion and acetylation. 3 or 4 react with glycosyl acceptor 5 to give disaccharide 6 or 7, respectively, with high α-selectivity. As amino sugars having 1,2-cis glycosidic bonds exist in many bioactive sugar chains such as heparin, application of these donors to bioactive oligosaccharide synthesis is promising.

References
- 1)Selective α-glycosylation and one-pot oligosaccharide synthesis involving 1,2-cis-glycosylation
References
Related Compounds
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.