Maximum quantity allowed is 999
CAS RN: 558-13-4 | 제품번호: T0038
Carbon Tetrabromide
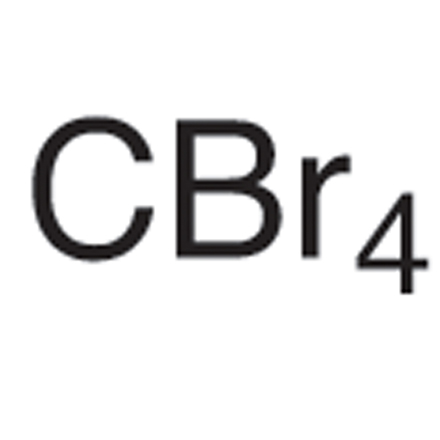
순도/분석 방법: >99.0%(GC)
- Tetrabromomethane
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
| Appearance | White powder to crystal |
| Purity(GC) | min. 99.0 % |
| Melting point | 91.0 to 95.0 °C |
| Solubility in Methanol | almost transparency |
| mp | 94 °C |
| bp | 190 °C |
| Solubility in water | Insoluble |
| Degree of solubility in water | 0.24 g/l 30 °C |
| 픽토그램 |



|
| 신호 워드 | Danger |
| 위험물 및 유해 등록 | H302 : Harmful if swallowed. H315 : Causes skin irritation. H318 : Causes serious eye damage. H371 : May cause damage to organs. H370 : Causes damage to organs. H372 : Causes damage to organs through prolonged or repeated exposure. H373 : May cause damage to organs through prolonged or repeated exposure. H336 : May cause drowsiness or dizziness. |
| 주의 사항 | P501 : Dispose of contents/ container to an approved waste disposal plant. P260 : Do not breathe dust/ fume/ gas/ mist/ vapors/ spray. P270 : Do not eat, drink or smoke when using this product. P271 : Use only outdoors or in a well-ventilated area. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P308 + P311 : IF exposed or concerned: Call a POISON CENTER/doctor. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. P304 + P340 + P312 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER/doctor if you feel unwell. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P403 + P233 : Store in a well-ventilated place. Keep container tightly closed. P405 : Store locked up. |
| RTECS # | FG4725000 |
| UN 번호 | UN2516 |
| 등급 | 6.1 |
| 포장 그룹 | III |
| HS 번호* | 2903.69-000 |

-
Used Chemicals
-
Procedure
-
To a mixture of 2-phenylethyl alcohol (0.196 mL, 1.64 mmol) and carbon tetrabromide (0.652 g, 1.96 mmol) in CH2Cl2 (8.2 mL) was added a solution of PPh3 (0.644 g, 2.46 mmol) in CH2Cl2 (3.3 mL) at 0 ºC. The reaction mixture was stirred at room temperature for 1 h, concentrated under reduced pressure, and purified by silica gel column chromatography (hexane:ethyl acetate = 7:3) to give (2-bromoethyl)benzene (0.290 g, 96% yield) as a colorless liquid.
-
Experimenter’s Comments
-
The reaction mixture was monitored by TLC (hexane:ethyl acetate = 4:1, Rf = 0.7).
-
Analytical Data
-
1H NMR (400 MHz, CDCl3); δ 7.36-7.20 (m, 5H), 3.78 (t, 2H, J = 11.2 Hz), 3.17 (t, 2H, J = 11.2 Hz).
13C NMR (101 MHz, CDCl3); δ 138.9, 128.6, 128.5, 126.9, 39.4, 32.9.
-
Lead Reference
-
- Hexabromoacetone and ethyl tribromoacetate: a highly efficient reagent for bromination of alcohol
- 1)Halo sugar nucleosides. III. Reactions for the chlorination and bromination of nucleoside hydroxyl groups
- 2)General 1,5-diene synthesis involving overall allyl alcohol coupling with geometrical and positional control
- Total synthesis of (+)-Lithospermic acid by asymmetric intramolecular alkylation via catalytic C-H bond activation
ApplicationBromination of Alcohols
References
ApplicationCorey-Fuchs Alkyne Synthesis Typical Procedure:
Typical Procedure:
Carbon tetrabromide (34.67 g, 104.5 mmol) and anhydrous CH2Cl2 (175 mL) are added to a 500 mL round bottom flask equipped with a magnetic stir bar and septum under N2 atmosphere. The reaction flask is cooled to 0 ℃ with an ice bath for 10 min. Triphenylphosphine (54.84 g, 209.1 mmol) is added in portions over 5 min, and the dark red solution is stirred at 0 ℃ for 30 min. 3,4-Dimethoxybenzaldehyde (17.37 g, 104.5 mmol) is added and the mixture is stirred at 0 ℃ for 1 h. A 1 : 1 mixture of H2O : brine is added and the layers are separated. The aqueous layer is extracted with a 1 : 1 mixture of hexanes : CH2Cl2, and the combined organic layers are dried, filtered, and concentrated under reduced pressure. The crude material is chromatographed on SiO2 (0-15% EtOAc/hexanes) to afford the dibromide 1 (29.37 g, Y. 88%) as a yellow oil.
Then, 1 (4.41 g, 13.79 mmol, 1.0 equiv) and THF (45 mL) are added to a 250 mL round bottom flask equipped with a magnetic stir bar and septum, and the reaction flask is cooled to -78 ℃ under N2 atmosphere. 2.5M n-BuLi solution in hexane (11.6 mL) is added slowly via syringe and the reaction solution is stirred at -78 ℃ for 45 min and then at 0 ℃ for 45 min. The flask is recooled to -78 ℃, and freshly distilled methyl chloroformate (1.1 mL, 1.3 mmol, 1.0 equiv) is added slowly via syringe. The mixture is stirred at -78 ℃ for 10 min, then 0 ℃ for 1 h. Saturated aqueous NH4Cl solution is added, and the layers are separated. The aqueous layer is extracted with Et2O, and the combined organic layers are dried, filtered and concentrated under reduced pressure. The residue is chromatographed (SiO2, 2-20% EtOAc / hexanes) to afford the desired alkyne 2 (2.83 g, Y. 93%) as a white solid.References
PubMed Literature
SDS
요청한 SDS를 사용할 수 없습니다.
번거롭게 해드려 죄송하지만 이 양식 보다 문의 해주십시오.
규격표
시험성적서, 각종 증명서
샘플 시험성적서
본 제품의 샘플시험성적서는 현재 준비되어 있지 않습니다.
분석 차트

죄송합니다만 찾으시는 분석차트는 없습니다.




