Maintenance Notice (3:00 AM - 8:30 AM December 13, 10:30 PM December 13 - 12:40 AM December 14 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [Product Highlights] Catechol-containing Methacrylate Monomer for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
A New Julia-Type Reagent for Methylenation Reactions
No.170(July 2016)
Ando et al. have reported that 1-methyl-2-(methylsulfonyl)benzimidazole (1) can be used as a methylene source and applied to the Julia-Kocienski-type methylenation reaction. 1 successfully reacts with aldehydes and ketones in the presence of a base at room temperature to afford the desired methylene derivatives. In this reaction, various ketones such as α,β-unsaturated ketones and sterically hindered ketones are applicable. In addition, after the reaction, formed byproducts of a benzimidazole derivative can be easily removed by the extraction.
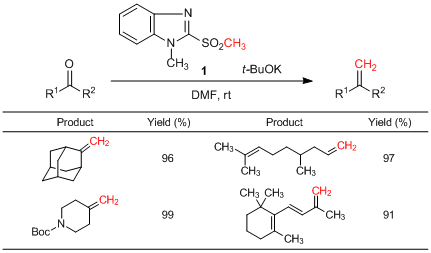
References
- Practical methylenation reaction for aldehydes and ketones using new Julia-type reagents
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.