Published TCIMAIL newest issue No.200
Maximum quantity allowed is 999
| Einheit | Stückpreis | Belgien | Japan* | Menge |
Versandinformationen

|
|---|---|---|---|---|---|
| 250MG |
€79.00
|
Kontaktieren Sie uns | Kontaktieren Sie uns |
|
|
| 1G |
€214.00
|
Versand innerhalb von 3 Wochen nach der Bestellung | 7 |
|
*Lagerbestand in Belgien: Versand am selben Tag
*Lagerbestand in Japan: Bitte prüfen Sie die Versandsimulation für voraussichtliche Lieferzeiten. (ausgenommen regulierte Produkte und Trockeneissendungen)
| Artikel # | B3161 |
Reinheit / Analysenmethode 
|
>98.0%(T) |
| Summenformel / Molekülmasse | C__2__4H__5__4P__2Pd = 511.06 |
| Physikalischer Zustand (20 °C) | Solid |
Lagerungstemperatur 
|
Frozen (<0°C) |
| Unter Inertgas lagern | Store under inert gas |
| Zu vermeidende Bedingungen | Air Sensitive,Heat Sensitive |
Verpackung und Behälter 
|
1G-Glass Bottle with Plastic Insert (Bild ansehen), 250MG-Glass Bottle with Plastic Insert (Bild ansehen) |
| CAS RN | 53199-31-8 |
| Reaxys Registrierungsnummer | 14300595 |
| PubChem-Stoff-ID | 87560386 |
| MDL-Nummer | MFCD03094580 |
| Appearance | White to Yellow to Orange powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
| HS-Nr. (Import / Export) (TCI-E) | 2843909000 |
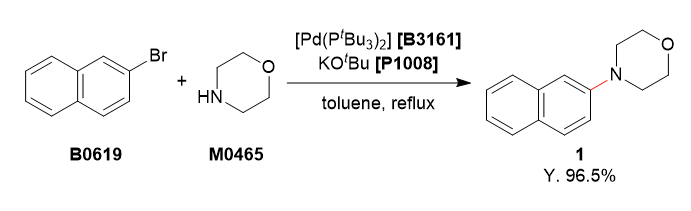
To a 4-necked 200 mL flask was charged with 2-bromonaphthalene (5.20 g, 25.1 mmol, 1.0 equiv.), morpholine (3.27 mL, 37.5 mmol, 1.5 equiv.) and degassed toluene (75 mL). To this solution was added bis(tri-tert-butylphosphine)palladium(0) (256 mg, 0.501 mmol, 2.0 mol%) and potassium tert-butoxide (4.21 g, 37.5 mmol, 1.5 equiv.). The reaction mixture was refluxed for 3 h under argon atmosphere. The reaction mixture was cooled to room temperature and washed with water (50 mL). The organic layer was separated and the aqueous layer was extracted with ethyl acetate (50 mL). The combined organic layers were washed with brine (50 mL), dried over Na2SO4 and filtered. The solvent was removed under reduced pressure. The residue was purified by column chromatography (eluent: hexane/ethyl acetate, 85/15→70/30) to obtain 1 as a white solid (5.17 g, 96.5%).
The reaction mixture was monitored by TLC (hexane/ethyl acetate = 9/1, Rf = 0.70).
1H NMR (400 MHz, CDCl3); δ 7.76–7.69 (m, 3H), 7.42 (t, J = 7.2 Hz, 1H), 7.31 (t, J = 6.8 Hz, 1H), 7.25–7.28 (m, 1H), 7.13 (s, 1H), 3.92 (t, J = 4.8 Hz, 2H), 3.27 (t, J = 4.8 Hz, 2H).
13C NMR (101 MHz, CDCl3); δ 129.0, 127.6, 127.0, 126.5, 123.7, 119.1, 110.3, 67.1, 50.0.
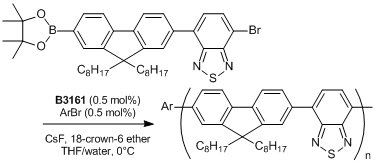

Typical Procedure: An ampoule is charged with the thiocarbamate (0.442 mmol) and anhydrous degassed toluene (4 mL) is added via gastight syringe under nitrogen with stirring. A J. Youngs valve is fitted and the ampoule is placed into an oil bath pre-equilibrated at 100℃. After ca. 5 minutes the valve is removed and Pd(t-Bu3P)2 (2 mol%, 0.00884 mmol) is added as a solid, the valve is then replaced and the reaction mixture heated for 2.5 hours. A sample of the reaction mixture is removed via gastight syringe and found to contain the desired product (>99%).
Das angeforderte SDB ist nicht verfügbar.
Bitte Kontaktieren Sie uns für mehr Informationen.
Ein Muster-AZ für dieses Produkt ist zur Zeit nicht verfügbar.

Das angeforderte Analysediagramm ist nicht verfügbar. Wir entschuldigen uns für die Unannehmlichkeiten.