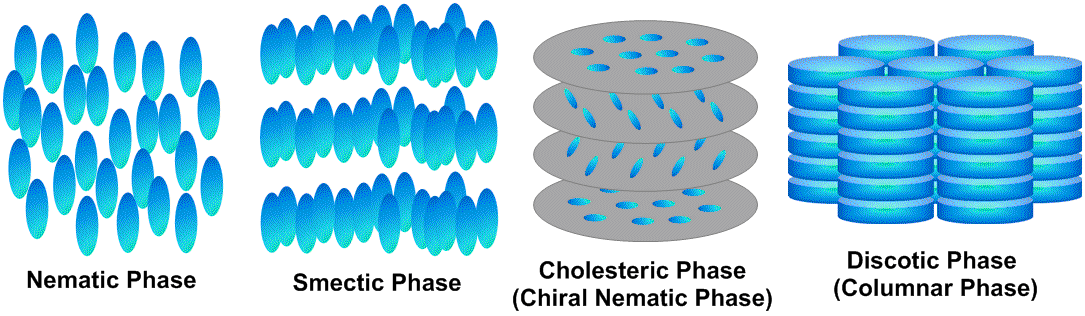
(1) Nematic phase
Calamitic shaped molecules are oriented one-dimensionally. The individual molecule can be relatively movable along the long axis direction. This phase belongs to the most flexible liquid crystal with large fluidity and small viscosity. Calamitic shaped cyanobiphenyls with large dielectric anisotropy (Δε) enable control of the molecular orientation by applying an electrical field. A liquid crystal display of a twisted nematic (TN) system3) is fabricated from a nematic liquid crystal.
(2) Smectic phase
There is a two-dimensional layered structure caused by more positional limitations compared with that of a nematic phase. A smectic phase is harder than a nematic phase, because the movable range of the unit molecules is relatively narrow. A nematic phase sometimes changes to a smectic phase by decreasing the temperature. Diversity of the layered structures demonstrates many kinds of smectic phases.
(3) Cholesteric phase (Chiral nematic phase)
This phase is usually observed from cholesterol derivatives. The unit molecules are oriented one-dimensionally similar to a normal nematic phase, but the molecular orientation shows a twisted helical arrangement between layers. This is due to an asymmetric carbon (chiral center) in the cholesterol molecule. Accordingly, a cholesteric phase is called a chiral nematic phase. This chiral phase exhibits optical rotation, selective optical scattering, circular polarization, and dichroism. Recently, a research development on a ‘blue phase’ received much attention.4) This phase is observed between temperatures of the cholesteric phase and an isotropic liquid. One difficulty is that we can find the blue phase in a narrow temperature range of 1-2 degrees. However, one can widen the temperature range more than several dozens of degrees, when a polymer slightly forms in the blue phase (polymer-stabilized blue phase).5)
(4) Discotic phase
Formation of a discotic phase requires a discotic aromatic molecule such as phthalocyanine,6) triphenylene,7) hexabenzocoronene8) and so on, although nematic and smectic phases require calamitic molecules. A discotic molecule usually forms a one-dimensional columnar structure (columnar phase) by stacking the molecules. A research area on organic electronics focuses on the discotic phase, because electrical conduction may occur along the molecular stacking direction. On the other hand, a rare example was reported that a chemical modification of a discotic molecule provided a three-dimensionally stacked cubic phase,9) whereas discotic molecules normally stack one-dimensionally.
Published TCIMAIL newest issue No.200