Light and flexible organic semiconductor materials are promising for foldable electronic circuits1) and implantable biometric sensors,2) although such electronic devices are hardly obtained from silicon-based semiconductors. We have developed a printed electronics giving large scale and highly precise devices on flexible substrates (eg. paper and film) by a printing method thanks to solubility of organic materials. The printing method is an efficient technology for mass production and low cost production of a semiconducting device.3) Further advantage using an organic material is that one can precisely control electrical properties and processing characteristics by chemical modification of the material, because organic materials are structurally diverse.
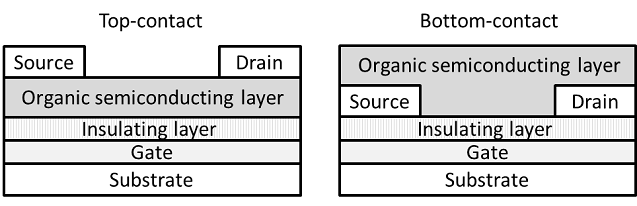
One functional parameter for evaluation of an organic semiconductor is mobility (μ) that shows how fast holes or electrons move in the semiconducting layer. We need an organic material with high mobility for highly drivable electronic circuits. The mobility can be measured from an organic field-effect transistor (OFET). There are TOF and TRMC methods for the measurement. The OFET can be fabricated by an organic semiconductor layer, insulating layer, source, drain and gate electrodes. In addition to the mobility value, we can determine fundamental OFET characteristics such as minimum drive voltage and drive stability. There are several types of OFET device structures with top-contact and bottom-contact systems. Furthermore, there is also a vertical transistor in which a carrier is movable vertically.
There are p-type organic semiconductors with hole carriers, n-type organic semiconductors with electron carriers and ambipolar organic semiconductors with both hole and electron carriers. Among them, there are small molecule p-type organic semiconductors; rubrene and pentacene as acene series, dinaphthothienothiophene (DNTT) and benzothienobenzothiophene (BTBT) as heteroacene series, oligothiophene series and porphyrin series. A category of n-type small molecule semiconductor involves perylene tetracarboxydiimide (PTCDI) and tetracyanoquinodimethane (TCNQ), and fullerenes. A polymer-based organic transistor also has been developed by using polythiophene, polyfluorene, and a donor-acceptor type polymer. A mobility value obtained from a solution-processed OFET has been normally lower than that obtained from a vapor deposited one, thus solubility is incompatible with mobility so far. However, an excellent solution-processible device was recently developed by soluble organic materials with high mobility.4-7) Further synthetic and process developments of organic materials may provide an efficient solution-processible device.
One characteristic of organic semiconductors is flexibility. Liquid crystal organic semiconductors also receive attention, because they are more flexible than the usual crystalline and amorphous organic semiconductors.8,9) The liquid crystal organic semiconductor shows carrier mobility and self-assembly, in which the liquid crystal molecule spontaneously undergoes an orientation. Moreover, one can control the molecular orientation by applying an electrical field, thanks to flexibility of the liquid crystal molecule.
Topics
References
- 1) M. Kaltenbrunner, T. Sekitani, J. Reeder, T. Yokota, K. Kuribara, T. Tokuhara, M. Drack, R. Schwödiauer, I. Graz, S. Bauer-Gogonea, S. Bauer, T. Someya, Nature 2013, 499, 458.

- 2) K. Kuribara, H. Wang, N. Uchiyama, K. Fukuda, T. Yokota, U. Zschieschang, C. Jaye, D. Fischer, H. Klauk, T. Yamamoto, K. Takimiya, M. Ikeda, H. Kuwabara, T. Sekitani, Y.-L. Loo, T. Someya, Nat. Commun. 2012, 3, 723.

- 3) K. Suganuma, in Introduction to Printed Electronics, Springer, New York, 2014, 124, 148.
- 4) T. Okamoto, C. Mitsui, M. Yamagishi, K. Nakahara, J. Soeda, Y. Hirose, K. Miwa, H. Sato, A. Yamano, T. Matsushita, T. Uemura, J. Takeya, Adv. Mater. 2013, 25, 6392.

- 5) H. Ebata, T. Izawa, E. Miyazaki, K. Takimiya, M. Ikeda, H. Kuwabara, T. Yui, J. Am. Chem. Soc. 2007, 129, 15732.

- 6) G. Kim, S.-J. Kang, G. K. Dutta, Y.-K. Han, T. J. Shin, Y.-Y. Noh, C. Yang, J. Am. Chem. Soc. 2014, 136, 9477.

- 7) H. Minemawari, T. Yamada, H. Matsui, J. Tsutsumi, S. Haas, R. Chiba, R. Kumai, T. Hasegawa, Nature 2011, 475, 364.

- 8) Review: E.-K. Fleischmann, R. Zentel, Angew. Chem. Int. Ed. 2013, 52, 8810.

- 9) M. A. Alam, J. Motoyanagi, Y. Yamamoto, T. Fukushima, J. Kim, K. Kato, M. Takata, A. Saeki, S. Seki, S. Tagawa, T. Aida, J. Am. Chem. Soc. 2009, 131, 17722.

