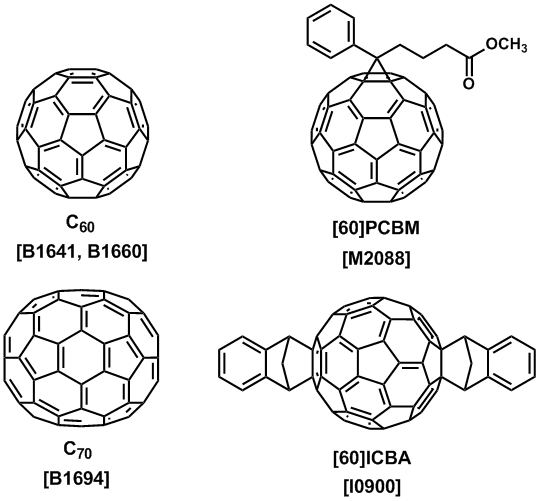
Addition reactions and other chemical modifications of fullerenes easily produce fullerene derivatives. Precise structure analyses of these derivatives are possible because they are molecular species. Non-derivatized fullerenes are poorly soluble in similarity to the other nanocarbon materials. However, we can introduce soluble functional groups to form solution-processible electronic materials. Phenyl-C61-butyric acid methyl ester ([60]PCBM) and indene-C60 bisadduct (ICBA) are useful organic semiconductors for fabricating a solution-processible electronic device.5,6) These fullerene derivatives are n-type organic semiconductors for organic photovoltaics (OPV) by mixing with a p-type conjugated polymer.7) An application of a fullerene derivative for organic transistors was also reported.8) A complexation of C60 with tetrakis(dimethylamino)ethylene (TDAE) gives a charge transfer complex (TDAE-C60), which is an organic magnet at low temperature.9)
Although a chemical modification of the outer surface of fullerene provides PCBM or ICBA, we can introduce a small component to the inner side of fullerene. For instance, fullerenes can encapsulate a metal atom on the inner side, when the fullerenes are produced in the presence of the metal. This is the so-called metal-encapsulated fullerene described as MC60. The encapsulation modifies the electronic state10) and chemical reactivity of fullerene.11) On the other hand, water-encapsulated fullerene (H2OC60) was also reported. A publication described that an organic synthetic procedure gave open-caged C60 which could then encapsulate one water molecule. A following chemical modification could close the cage to from water-encapsulated fullerene H2OC60.12)
Published TCIMAIL newest issue No.197



![Fullerene C60 (purified by sublimation) [for organic electronics] Fullerene C60 (purified by sublimation) [for organic electronics]](/medias/F1232.jpg?context=bWFzdGVyfHJvb3R8Mzg4ODd8aW1hZ2UvanBlZ3xhR1V6TDJobE5TODVNVGMyTkRjNE1qZzFPRFUwTDBZeE1qTXlMbXB3Wnd8NWZhZWYzYTVhNWRiY2U5Y2NlNzk5ZGU3MzRhN2QzNDEwMDk4OWJhOWNkZDMzZjczYWRhNWE3MzQ3ZTlhZTBkNQ)
![[6,6]-Phenyl-C61-butyric Acid Methyl Ester [for organic electronics] [6,6]-Phenyl-C61-butyric Acid Methyl Ester [for organic electronics]](/medias/P2682.jpg?context=bWFzdGVyfHJvb3R8NDk5MjV8aW1hZ2UvanBlZ3xhRFU1TDJnME5TODRPVE15TVRRME56QTVOall5TDFBeU5qZ3lMbXB3Wnd8NTU1ZTExOTA3MjFjYmRhYjIyMzAyY2Y0ZGM0ZjYzNjlhOTc4ZGY2ZDJiYjU0ZjUxZmIxNTQxZGI1MWY2NDNjMg)
![[6,6]-Phenyl-C61-butyric Acid Methyl Ester [6,6]-Phenyl-C61-butyric Acid Methyl Ester](/medias/M2088.jpg?context=bWFzdGVyfHJvb3R8NTAwMDZ8aW1hZ2UvanBlZ3xhR1JtTDJnNU15ODRPVE14TkRrNU56WTVPRGcyTDAweU1EZzRMbXB3Wnd8MGUwZjIyZTEzMWM5MWYwMTU5ZjRlZWJmYzY4ZGQ4NGRjZTYxNWZlYTNkNGU2ZjE3NWRhNTUwM2NjZDVhNzcxOA)
![Fullerene C70 (purified by sublimation) [for organic electronics] Fullerene C70 (purified by sublimation) [for organic electronics]](/medias/F1233.jpg?context=bWFzdGVyfHJvb3R8NTc2MTN8aW1hZ2UvanBlZ3xhREEwTDJoa015ODRPVE13TnpJME1qZ3lNems0TDBZeE1qTXpMbXB3Wnd8MjRiMGMxYWUxNzAyZmYyNTFkODViMTFkMjUxOWViMjAyN2QwMGU1ZmRhMjkzMTBiODlhMjViNjJhMjIzZTUwZQ)
![[6,6]-Phenyl-C71-butyric Acid Methyl Ester (mixture of isomers) [for organic electronics] [6,6]-Phenyl-C71-butyric Acid Methyl Ester (mixture of isomers) [for organic electronics]](/medias/P2683.jpg?context=bWFzdGVyfHJvb3R8NTA4Njd8aW1hZ2UvanBlZ3xhRGs0TDJnME5DODRPVE15TVRRME56YzFNVGs0TDFBeU5qZ3pMbXB3Wnd8ODY2ZWFlY2Y5NjUxN2E5ODA5NWRkZTkwMzViMWJkYjM5ZmMwYmI0MzAxMWJiNzRmNGUxMDE3Zjc2MTFhN2E4Yw)


![N,2-Diphenyl[60]fulleropyrrolidine (contains 5% Hexane at maximum) N,2-Diphenyl[60]fulleropyrrolidine (contains 5% Hexane at maximum)](/medias/D5757.jpg?context=bWFzdGVyfHJvb3R8NDU5MzZ8aW1hZ2UvanBlZ3xhR0ppTDJnNFppODRPVE13TkRVd09EWTJNakEyTDBRMU56VTNMbXB3Wnd8ZTk1ZTBmODllOTNjYTA0MDcyNjgwZjc3NWFhYzg0MjAxZTc2MGI2YmJjNDlhMTU2ODQ5NDhjZWFjMzM1ZGFiOQ)
![[6,6]-Phenyl-C71-butyric Acid Methyl Ester (mixture of isomers) [6,6]-Phenyl-C71-butyric Acid Methyl Ester (mixture of isomers)](/medias/M2550.jpg?context=bWFzdGVyfHJvb3R8NTAwNTV8aW1hZ2UvanBlZ3xhRGRtTDJnek5DODRPVE14TlRnNU9UUTNOREl5TDAweU5UVXdMbXB3Wnd8YzU4ZDkwMDkzMDQ3NTI4MjI2NTE4YmVlNWZiNDc2YTNjYjVlMTNjNTY3ZDJmNGM0ZTgxMDM3M2Y5MGJlZDVjNQ)

![[6,6]-Phenyl-C61-butyric Acid Butyl Ester [6,6]-Phenyl-C61-butyric Acid Butyl Ester](/medias/P2013.jpg?context=bWFzdGVyfHJvb3R8NDgzNzB8aW1hZ2UvanBlZ3xhREF5TDJoaU1TODRPVE15TVRFME9ESTFNalEyTDFBeU1ERXpMbXB3Wnd8Y2IyYjc5NWZhZTU5Y2U0OTQxNjkyZDQwNjNhZDRhMTc3NDFkNmE2ZDZhNTcxZmYwMGIyMjJjYWMxYWRiOGU0Yg)