Maximum quantity allowed is 999
Please select the quantity
CAS RN: 346440-54-8 | Product Number: B4138
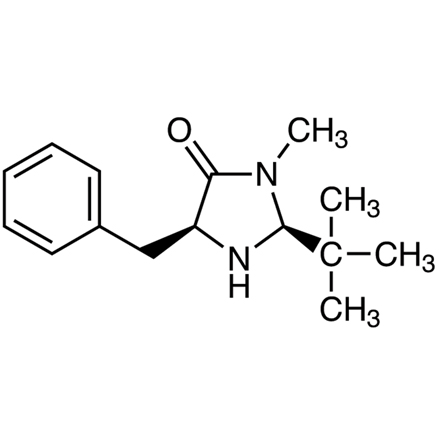
(2S,5S)-(-)-2-tert-Butyl-3-methyl-5-benzyl-4-imidazolidinone
Purity: >97.0%(GC)
Synonyms:
- (2S,5S)-(-)-5-Benzyl-2-tert-butyl-3-methyl-4-imidazolidinone
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 200MG |
$93.00
|
1 | 0 | Contact Us | |
| 1G |
$360.00
|
23 | 1 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | B4138 |
Purity / Analysis Method 
|
>97.0%(GC) |
| Molecular Formula / Molecular Weight | C__1__5H__2__2N__2O = 246.35 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
Packaging and Container 
|
1G-Glass Bottle with Plastic Insert (View image), 200MG-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 346440-54-8 |
| Reaxys Registry Number | 6480916 |
| PubChem Substance ID | 354334237 |
| MDL Number | MFCD03426982 |
Specifications
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Optical purity(LC) | min. 98.0 ee% |
| Melting point | 97.0 to 101.0 °C |
Properties (reference)
| Melting Point | 100 °C |
| Specific Rotation | -60° (C=1,MeOH) |
GHS
Related Laws:
Transport Information:
| H.S.code* | 2933.29-000 |
Application
Imidazolidinone Derivatives for Versatile Asymmetric Reactions

References
- 1) The First Enantioselective Organocatalytic Mukaiyama-Michael Reaction: A Direct Method for the Synthesis of Enantioenriched γ-Butenolide Architecture
- 2) Enantioselective organocatalytic epoxidation using hypervalent iodine reagents
- 3) Enantioselective Organocatalytic Singly Occupied Molecular Orbital Activation: The Enantioselective α-Enolation of Aldehydes
- 4) Enantioselective Organocatalytic Intramolecular Diels−Alder Reactions. The Asymmetric Synthesis of Solanapyrone D
- 5) Total Synthesis of (−)-Spinosyn A via Carbonylative Macrolactonization
PubMed Literature
Product Articles
[Product Highlights] Imidazolidinone Derivatives for Versatile Asymmetric ReactionsProduct Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.



