Maintenance Notice (10:30 PM August 23 - 3:30 AM August 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Life Science News August 2025 | [Product Highlights] Puberulic Acid: A Compound with... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
请选择数量
CAS RN: 873779-78-3 | 产品编码: C2422
Chloro(1,3-dimesitylimidazol-2-ylidene)copper(I)
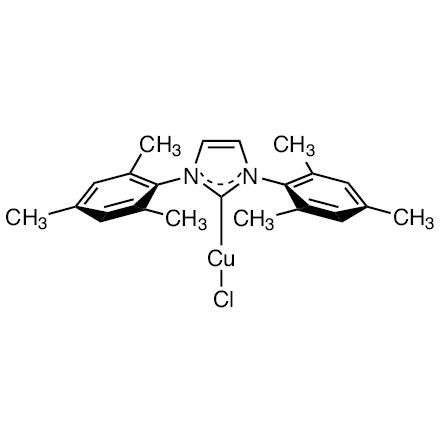
| 产品编码 | C2422 |
纯度/分析方法 
|
>97.0%(T) |
| 分子式/分子量 | C__2__1H__2__4ClCuN__2 = 403.43 |
| 外观与形状(20°C) | 固体 |
储存温度 
|
冷冻 (<0°C) |
| 应避免的情况 | 加热 |
包装和容器 
|
1G-Glass Bottle with Plastic Insert (查看图片), 200MG-Glass Bottle with Plastic Insert (查看图片) |
| CAS RN | 873779-78-3 |
| PubChem物质ID | 125307392 |
技术规格
| Appearance | White to Light yellow powder to crystal |
| Purity(Chelometric Titration) | min. 97.0 % |
物性(参考值)
| 熔点 | 277 °C |
GHS
相关法规
运输信息
| HS编码* | 2933.29-000 |
应用
N-Heterocyclic Carbene (NHC) Copper Complex Catalyst
References
- Efficient boron?copper additions to aryl-substituted alkenes promoted by NHC?based catalysts. enantioselective Cu-catalyzed hydroboration reactions
- Copper(I) 1,2,3-triazol-5-ylidene complexes as efficient catalysts for click reactions of azides with alkynes
- Allylic substitution reactions with Grignard reagents catalyzed by imidazolium and 4,5-dihydroimidazolium carbene?CuCl complexes
应用
(IMes)-copper complex catalyzed methylenation of cinnamaldehydes

Typical procedure (R = H):
To a solution of (IMes)-Cu complex catalyst (20.2 mg, 0.050 mmol) and triphenylphosphine (288 mg, 1.10 mmol) in dioxane (10 mL) at 25 °C is added 2-propanol (84 µL, 1.1 mmmol) followed by trans-cinnamaldehyde (125 µL, 1.00 mmol) and the trimethylsilyldiazomethane solution (1.4–2.0 mmol) under inert atmosphere. The resulting mixture is then heated at 60 °C for 2 h. Afterward, 3% H2O2 (10 mL) is added and the organic layer is extracted with ether (3 x 20 mL). The combined organic layers are washed with brine (2 x 20 mL) and dried over MgSO4. The filtrate is removed under reduced pressure and the residue is purified by flash chromatography on silica gel (eluent: 1% EtOAc/hexanes) to give 1-phenyl-1,3-butadiene (100 mg, Y.77%) as a colorless oil.
To a solution of (IMes)-Cu complex catalyst (20.2 mg, 0.050 mmol) and triphenylphosphine (288 mg, 1.10 mmol) in dioxane (10 mL) at 25 °C is added 2-propanol (84 µL, 1.1 mmmol) followed by trans-cinnamaldehyde (125 µL, 1.00 mmol) and the trimethylsilyldiazomethane solution (1.4–2.0 mmol) under inert atmosphere. The resulting mixture is then heated at 60 °C for 2 h. Afterward, 3% H2O2 (10 mL) is added and the organic layer is extracted with ether (3 x 20 mL). The combined organic layers are washed with brine (2 x 20 mL) and dried over MgSO4. The filtrate is removed under reduced pressure and the residue is purified by flash chromatography on silica gel (eluent: 1% EtOAc/hexanes) to give 1-phenyl-1,3-butadiene (100 mg, Y.77%) as a colorless oil.
References
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)






