Maximum quantity allowed is 999
请选择数量
CAS RN: 136572-09-3 | 产品编码: I0714
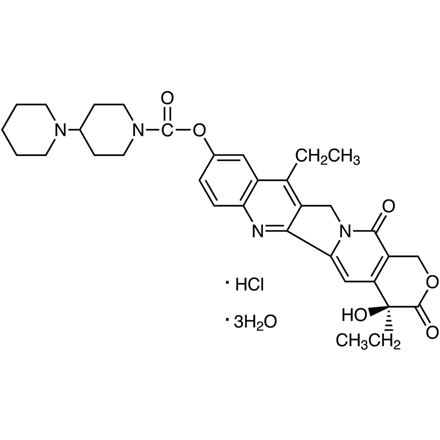
Irinotecan Hydrochloride Trihydrate
| 产品编码 | I0714 |
纯度/分析方法 
|
>98.0%(T)(HPLC) |
| 分子式/分子量 | C__3__3H__3__8N__4O__6·HCl·3H__2O = 677.20 |
| 外观与形状(20°C) | 固体 |
储存温度 
|
冷藏 (0-10°C) |
| 应避免的情况 | 加热 |
包装和容器 
|
100MG-Glass Bottle with Plastic Insert (查看图片) |
| CAS RN | 136572-09-3 |
相关CAS RN 
|
100286-90-6&97682-44-5 |
| Reaxys-RN | 4838283 |
| PubChem物质ID | 87560270 |
| Merck Index (14) | 5091 |
| MDL编号 | MFCD01765731 |
技术规格
| Appearance | White to Orange to Green powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(Nonaqueous Titration) | min. 98.0 % |
| Specific rotation [a]20/D | +22.0 to +26.0 deg(C=1, methanol) |
| Water | 7.0 to 10.0 % |
物性(参考值)
| 沸点 | 257 °C |
| 比旋光度 [α]D | 24° (C=1,MeOH) |
| 溶解性(可溶于) | 甲醇 |
GHS
| 象形图 |


|
| 信号词 | Danger |
| 危险性说明 | H302 : Harmful if swallowed. H372 : Causes damage to organs through prolonged or repeated exposure. |
| 防范说明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P260 : Do not breathe dust/ fume/ gas/ mist/ vapors/ spray. P270 : Do not eat, drink or smoke when using this product. P264 : Wash skin thoroughly after handling. P314 : Get medical advice/ attention if you feel unwell. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. |
相关法规
| RTECS# | DW1060750 |
运输信息
| HS编码* | 2933.99-000 |
应用
Irinotecan hydrochloride (CPT-11): A Water Soluble Topoisomerase-I Inhibitor with Enhanced Activity
Irinotecan hydrochloride (CPT-11) is a derivative of 7-ethylcamptothecin (SN-22) [E0781] or Camptothecin (CPT) [C1495]. Irinotecan hydrochloride has greater water solubility and enhanced topoisomerase-I inhibitory activity than theirs. Irinotecan, a prodrug, is converted to a biologically active metabolite 7-ethyl-10-hydroxy-camptothecin (SN-38) [E0748] by a carboxylesterase-converting enzyme. Camptotecin and its derivatives interfere with DNA synthesis by inhibiting the enzyme topoisomerase-I. In order to prevent and correct of topological problems caused by the DNA double helix, topoisomerase-I catalyzes the relaxation of negatively supercoiled DNA, the knotting and unknotting DNA and the linking complementary rings of single-stranded DNA into double-stranded rings. Then the inhibition action induces breaks in single strand DNA. Eventually, this leads to double-strand DNA breaks and apoptosis or cell death. (The product is for research purpose only.)
References
- Synthesis and antitumor activity of 20(S)-camptothecin derivatives: carbamate-linked, water-soluble derivatives of 7-ethyl-10-hydroxycamptothecin
- Identification of a new metabolite of CPT-11 (irinotecan): pharmacological properties and activation to SN-38
- Clinical pharmacokinetics and metabolism of irinotecan (CPT-11) (a review)
- UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity
- The camptothecins (a review)
- Pharmacogenetics of irinotecan metabolism and transport: An update
参考文献
文档
技术文章
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱

请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。






