Maximum quantity allowed is 999
请选择数量
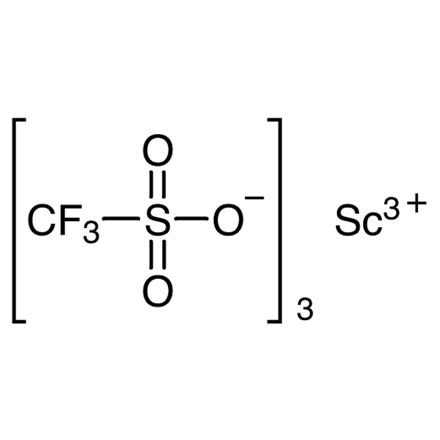
CAS RN: 144026-79-9 | 产品编码: T1663
Scandium(III) Trifluoromethanesulfonate
| 产品编码 | T1663 |
纯度/分析方法 
|
>98.0%(T) |
| 分子式/分子量 | C__3F__9O__9S__3Sc = 492.15 |
| 外观与形状(20°C) | 固体 |
储存温度 
|
室温 (15°C以下阴凉干燥处) |
| 储存在惰性气体下 | 存放于惰性气体之中 |
| 应避免的情况 | 湿气 (吸湿) |
包装和容器 
|
1G-Glass Bottle with Plastic Insert (查看图片) |
| CAS RN | 144026-79-9 |
| Reaxys-RN | 8510151 |
| PubChem物质ID | 87577404 |
| MDL编号 | MFCD00192433 |
技术规格
| Appearance | White to Almost white powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
物性(参考值)
| 水溶性 | 可溶 |
GHS
| 象形图 |

|
| 信号词 | Warning |
| 危险性说明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防范说明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
相关法规
运输信息
| HS编码* | 2846.90-000 |
应用
Acetylation of Alcohols using Acetylating Reagents and Acid Catalysts
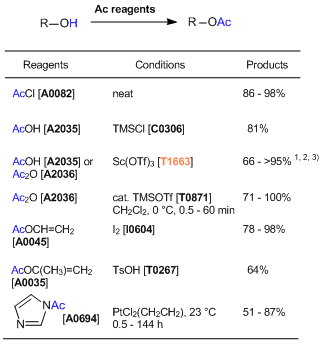
References
- 1)A Novel Method for the Preparation of Macrolides from ω-Hydroxycarboxylic Acids
- 2)An Efficient Esterification Reaction between Equimolar Amounts of Free Carboxylic Acids and Alcohols by the Combined Use of Octamethylcyclotetrasiloxane and a Catalytic Amount of Titanium(IV) Chloride Tris(trifluoromethanesulfonate)
- 3)Scandium Trifluoromethanesulfonate as an Extremely Active Lewis Acid Catalyst in Acylation of Alcohols with Acid Anhydrides and Mixed Anhydrides
- 4)Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
应用
Stereoinversion of tertiary alcohols to tertiary-alkyl isonitriles
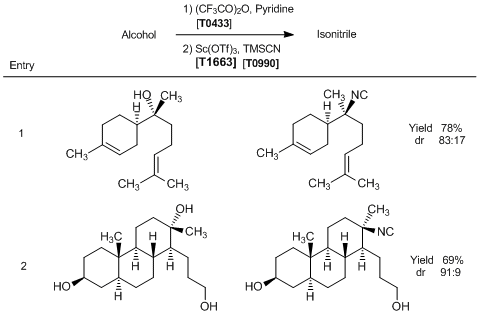
Typical Procedure (entry 1): A solution of (-)-α-bisabolol (22.2 mg, 0.1 mmol) in anhydrous dichloromethane (0.15 mL) is cooled to 0 °C and treated with anhydrous pyridine (31.6 mg, 0.4 mmol), followed by trifluoroacetic anhydride (42 mg, 0.2 mmol). The reaction mixture is stirred at 0 °C for 20 min and quenched with 1 N aqueous HCl. The resulting biphasic mixture is stirred vigorously at room temperature for 5 min and extracted twice with hexanes. The combined organic layers are washed with water followed by saturated aqueous NaHCO3, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The resulting crude trifluoroacetate (32.0 mg, 0.1 mmol) is dissolved in TMSCN (0.1 mL), cooled to 0 °C, and treated drop-wise with a solution of anhydrous Sc(OTf)3 (1.5 mg, 0.003 mmol) in TMSCN (0.1 mL). The reaction mixture is left at 3 °C for 18 h and quenched with tetramethylethylenediamine (7.5 µl, 0.05 mmol). The resulting solution is concentrated under reduced pressure, and the residue is purified by flash column chromatography on silica gel (eluent: 35% dichloromethane in hexanes) to give 7-isocyano-7,8-dihydro-α-bisabolene (18.0 mg, 78% yield, dr 83:17) as a light yellow oil.
References
应用
Reusable Lewis Acid
References
- A novel reusable Lewis acid catalyst in aldol and Michael reactions
- A novel reusable catalyst in Diels-Alder reaction
- A chiral scandium catalyst for enantioselective Diels-Alder reactions
- Aqueous reactions with Lewis acid and organometalic reagent
PubMed Literature
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱

请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。