Maximum quantity allowed is 999
Please select the quantity
CAS RN: | Product Number: C2637
Copper(II) on Chitosan (Cu 1.3mmol/g)
Purity:
Synonyms:
Product Documents:
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | C2637 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
Specifications
| Appearance | Green to Dark green to Dark blue powder to crystal |
| Loading | 1.2 to 1.5 mmol/g |
| Functionality test | to pass test |
Properties (reference)
GHS
Related Laws:
Transport Information:
| H.S.code* | 3822.19-000 |
Application
Copper (II) on Chitosan, a reagent for selective discrimination between 2-hydroxy and 2-deoxy sugars, an alternative test reagent for Fehling and Benedict reagents
Copper (II) on chitosan is a chitosan-supported copper (II) (about 1.3 mmol/g) developed by Inoue et al. as a reagent for selective detection of reducible organic compounds. The Cu (II) on chitosan can selectively detect reducible organic compounds bearing structures changeable to ene-diolate (e.g. glucose, ribose) in basic aqueous solution with the formation of copper (I) oxide (Cu2O) as a signal of the reduction by the ene-diolate.


(The color of Cu (II) on chitosan is subject to change to green after opening. But the green-colored Cu (II) on chitosan changes to dark blue which is suitable for this detection in basic aqueous solution, in the same way as a new reagent.)
Typical procedure (detection of a sugar): A test tube (15 mm inside diameters) is charged with copper (II) on chitosan (20 mg) and an aqueous solution of 0.5 mol/L NaOH (1 mL). At this point, the color of copper (II) on chitosan changes from blue to dark blue. Then, an aqueous solution of 10 mmol/L sugar (4 mL) is added, and the mixture is stirred at 70 to 80 °C for 5 min.
If it gives a positive result, red-brown to orange colored Cu2O on chitosan is observed (e.g. glucose and ribose). If it gives a negative result, black to blue colored CuO on chitosan is observed (e.g. 2-deoxyglucose and 2-deoxy ribose).
If it gives a positive result, red-brown to orange colored Cu2O on chitosan is observed (e.g. glucose and ribose). If it gives a negative result, black to blue colored CuO on chitosan is observed (e.g. 2-deoxyglucose and 2-deoxy ribose).
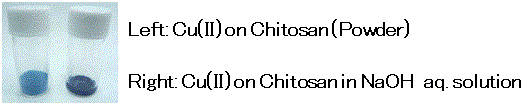

Using Cu (II) on chitosan, needs a lesser amount of Cu (II) than that of Fehling and Benedict reagents and leads to significantly reducing the reagent costs and waste chemicals. In addition, Cu (II) on chitosan is a powdery substance, which is easily handled and free from leaking in carrying and storage. Notably, Cu (II) on chitosan is suitable for science educational experiments such as a test for the detection of a reducing sugar yielded from starch hydrolysis.

References
- S. Ogura, M. Inoue, Kagaku to Kyoiku (Chemical Education) 2013, 61, 86. (Japanese)
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.






