Maximum quantity allowed is 999
请选择数量
CAS RN: | 產品號碼: C2637
Copper(II) on Chitosan (Cu 1.3mmol/g)
* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | C2637 |
| 外觀與形狀(20°C) | Solid |
儲存條件 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
產品規格
| Appearance | Green to Dark green to Dark blue powder to crystal |
| Loading | 1.2 to 1.5 mmol/g |
| Functionality test | to pass test |
性質
GHS
相關法規
運輸資料
| HS編碼* | 3822.19-000 |
Application
Copper (II) on Chitosan, a reagent for selective discrimination between 2-hydroxy and 2-deoxy sugars, an alternative test reagent for Fehling and Benedict reagents
Copper (II) on chitosan is a chitosan-supported copper (II) (about 1.3 mmol/g) developed by Inoue et al. as a reagent for selective detection of reducible organic compounds. The Cu (II) on chitosan can selectively detect reducible organic compounds bearing structures changeable to ene-diolate (e.g. glucose, ribose) in basic aqueous solution with the formation of copper (I) oxide (Cu2O) as a signal of the reduction by the ene-diolate.


(The color of Cu (II) on chitosan is subject to change to green after opening. But the green-colored Cu (II) on chitosan changes to dark blue which is suitable for this detection in basic aqueous solution, in the same way as a new reagent.)
Typical procedure (detection of a sugar): A test tube (15 mm inside diameters) is charged with copper (II) on chitosan (20 mg) and an aqueous solution of 0.5 mol/L NaOH (1 mL). At this point, the color of copper (II) on chitosan changes from blue to dark blue. Then, an aqueous solution of 10 mmol/L sugar (4 mL) is added, and the mixture is stirred at 70 to 80 °C for 5 min.
If it gives a positive result, red-brown to orange colored Cu2O on chitosan is observed (e.g. glucose and ribose). If it gives a negative result, black to blue colored CuO on chitosan is observed (e.g. 2-deoxyglucose and 2-deoxy ribose).
If it gives a positive result, red-brown to orange colored Cu2O on chitosan is observed (e.g. glucose and ribose). If it gives a negative result, black to blue colored CuO on chitosan is observed (e.g. 2-deoxyglucose and 2-deoxy ribose).
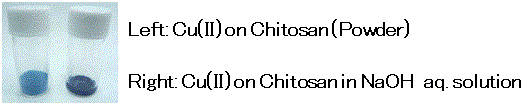

Using Cu (II) on chitosan, needs a lesser amount of Cu (II) than that of Fehling and Benedict reagents and leads to significantly reducing the reagent costs and waste chemicals. In addition, Cu (II) on chitosan is a powdery substance, which is easily handled and free from leaking in carrying and storage. Notably, Cu (II) on chitosan is suitable for science educational experiments such as a test for the detection of a reducing sugar yielded from starch hydrolysis.

References
- S. Ogura, M. Inoue, Kagaku to Kyoiku (Chemical Education) 2013, 61, 86. (Japanese)
產品文件 (部分產品的分析圖譜無法提供,敬請諒解。)
SDS
請選擇語言。
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
請輸入批號
輸入的批號不正確
示例 CoA
可下載CoA示例。注:該示例可能非最新批次的CoA。
目前沒有該產品的 CoA 示例。
分析圖譜

請輸入批號
輸入的批號不正確
很抱歉,您搜索的分析圖譜無法提供。






