Maximum quantity allowed is 999
请选择数量
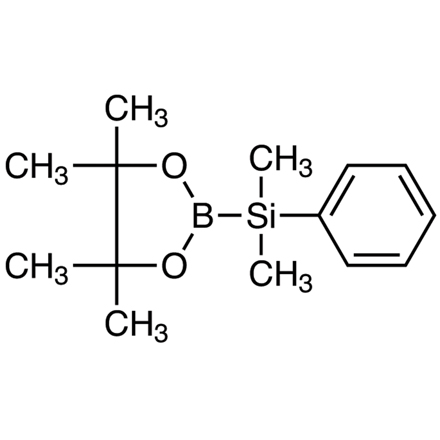
CAS RN: 185990-03-8 | 產品號碼: D4357
2-(Dimethylphenylsilyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | D4357 |
純度/分析方法 
|
>95.0%(GC) |
| 分子式 / 分子量 | C__1__4H__2__3BO__2Si = 262.23 |
| 外觀與形狀(20°C) | Liquid |
儲存條件 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| 儲存在惰性氣體下 | Store under inert gas |
| 應避免的情況 | Air Sensitive,Moisture Sensitive |
| CAS RN | 185990-03-8 |
| Reaxys-RN | 7585086 |
| PubChem Substance ID | 253661961 |
| MDL編號 | MFCD05664111 |
產品規格
| Appearance | Colorless to Light yellow to Light orange clear liquid |
| Purity(GC) | min. 95.0 % |
性質
| 沸點 | 120 °C/0.08 mmHg |
| 比重 | 0.97 |
| 折射率 | 1.50 |
GHS
相關法規
運輸資料
| HS編碼* | 2934.99-000 |
Application
Transition Metal-free Borylation
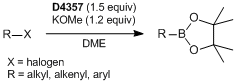
A Representative procedure for the boryl substitution reaction of aryl halide:
To a vial sealed with a screw cap containing a silicon-coated rubber septum is added potassium methoxide (0.6 mmol) under argon. It is connected to a vacuum/nitrogen manifold through a needle. DME (5 mL) and 1 (0.36 mmol) are added to the vial, then stirred for 10 min at 30 °C. 4-Bromoanisole (0.50 mmol) is added dropwise and stirred for 1 h. The solution is cooled to 0 °C followed by the addition of TBAF (1.0 M, 800 µL). The resultant solution is stirred for 3 h at 0 °C. After that, the mixture is added to the H2O (100 mL), then extracted with Et2O (50 mL). The organic layer is washed with water (50 mL x 2). The combined organic layer is then dried over MgSO4 followed by evaporation. The crude product is purified by boric acid-impregnated silica-gel column chromatography with 0–5% hexane/Et2O eluent to give the desired product (77% isolated yield) as a colorless oil.
To a vial sealed with a screw cap containing a silicon-coated rubber septum is added potassium methoxide (0.6 mmol) under argon. It is connected to a vacuum/nitrogen manifold through a needle. DME (5 mL) and 1 (0.36 mmol) are added to the vial, then stirred for 10 min at 30 °C. 4-Bromoanisole (0.50 mmol) is added dropwise and stirred for 1 h. The solution is cooled to 0 °C followed by the addition of TBAF (1.0 M, 800 µL). The resultant solution is stirred for 3 h at 0 °C. After that, the mixture is added to the H2O (100 mL), then extracted with Et2O (50 mL). The organic layer is washed with water (50 mL x 2). The combined organic layer is then dried over MgSO4 followed by evaporation. The crude product is purified by boric acid-impregnated silica-gel column chromatography with 0–5% hexane/Et2O eluent to give the desired product (77% isolated yield) as a colorless oil.
References
- Anomalous Reactivity of Silylborane: Transition-Metal-Free Boryl Substitution of Aryl, Alkenyl, and Alkyl Halides with Silylborane/Alkoxy Base Systems
考研文獻
產品文件 (部分產品的分析圖譜無法提供,敬請諒解。)




