Maximum quantity allowed is 999
CAS RN: 3240-34-4 | 產品號碼: I0330
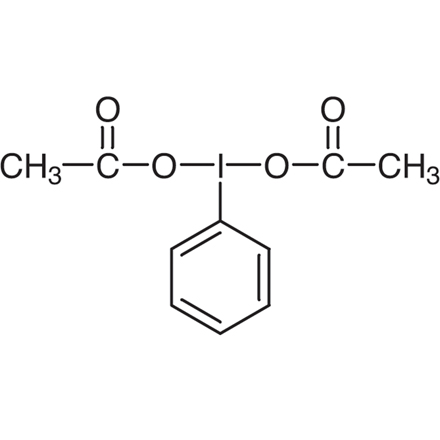
Iodobenzene Diacetate
* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | I0330 |
| 純度/分析方法 | >97.0%(T) |
| 分子式 / 分子量 | C__1__0H__1__1IO__4 = 322.10 |
| 外觀與形狀(20°C) | Solid |
| 儲存條件 | Room Temperature (Recommended in a cool and dark place, <15°C) |
| 儲存在惰性氣體下 | Store under inert gas |
| 應避免的情況 | Moisture Sensitive |
| CAS RN | 3240-34-4 |
| Reaxys-RN | 1879369 |
| PubChem Substance ID | 87571686 |
| SDBS (AIST Spectral DB) | 12662 |
| MDL編號 | MFCD00008692 |
| Appearance | White to Light yellow powder to crystal |
| Purity(Iodometric Titration) | min. 97.0 % |
| Solubility in Methanol | almost transparency |
| 熔點 | 158 °C(dec.) |
| 水溶性 | Insoluble |
| 溶解性(可溶於) | Methanol |
| 溶解性(不溶於) | Ether |
| 圖形表示 |

|
| 信號詞 | Warning |
| 危險性說明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防範說明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| RTECS # | DA3525000 |
| HS編碼* | 2931.90-000 |

-
Used Chemicals
-
Procedure
-
To a solution of 1-naphthalenemethanol (306 mg, 2.0 mmol) in dichloromethane (2 mL, 1.0 mol/L) was added TEMPO (31.2 mg, 0.20 mmol), PhI(OAc)2 (709mg, 2.2 mmol) and the mixture was stirred at room temperature for 4 hours. Dichloromethane (12.5 mL), saturated aqueous sodium thiosulfate solution (12.5 mL) was added and the mixture was stirred for 30 minutes. The organic layer was washed with saturated aqueous sodium bicarbonate solution (10 mL), brine (10 mL) and the organic layer was dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by column chromatography (ethyl acetate:hexane = 0:100 - 3:97 on silica gel) to give 1-naphthaldehyde as a yellow liquid (291 mg, 93%).
-
Experimenter’s Comments
-
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 1:9, Rf = 0.50).
-
Analytical Data
-
1-Naphthaldehyde
1H NMR (400 MHz, CDCl3); δ 10.41 (s, 1H), 9.26 (d, J = 8.9 Hz, 1H), 8.11 (d, J = 8.4 Hz, 1H), 8.01 (d, J = 6.8 Hz, 1H), 7.93 (d, J = 8.9 Hz, 1H), 7.74-7.57 (m, 3H).
-
Lead Reference
-
- 2-(Hydroxyimino)aldehydes: Photo- and Physicochemical Properties of a Versatile Functional Group for Monomer Design

-
Used Chemicals
-
Procedure
-
To a solution of 2-phenylimidazoline (500 mg, 3.4 mmol), potassium carbonate (520 mg, 3.8 mmol, 1.1 equiv) in DMSO (17 mL) was added iodobenzene diacetate (1.21 g, 3.8 mmol, 1.1 equiv) and stirred at rt for 18 hours. The residue was diluted with ethyl acetate (75 mL) and washed with saturated aqueous sodium bicarbonate solution (50 mL, twice) dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by column chromatography (on aminosilica gel, ethyl acetate:hexane = 0:1 - 1:0), giving 2-phenylimidazole as a white solid (340 mg,y. 69%).
-
Experimenter’s Comments
-
The reaction mixture was monitored by NH-TLC (ethyl acetate, Rf = 0.30).
-
Analytical Data
-
2-phenylimidazole
1H NMR (270 MHz, DMSO-d6); δ 12.5 (dr, 1H), 7.95-7.91 (m, 2H), 7.44 (t, 2H, J = 7.3 Hz), 7.33 (t, 1H, J = 7.3 Hz), 7.24 (s, 1H), 7.02 (s, 1H)
-
Lead Reference
-
- Expanded applicability of iridium(I) NHC/phosphine catalysts in hydrogen isotope exchange processes with pharmaceutically-relevant heterocycles

References
- G. Piancatelli, F. Leonelli, Org. Synth. 2006, 83, 18.
[Research Articles] Synthesis of 1,2-Diamine Derivatives Using a Rhodium Catalyst
SDS
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
示例 CoA
目前沒有該產品的 CoA 示例。
分析圖譜
很抱歉,您搜索的分析圖譜無法提供。




