Product Document Searching Made Easy by 2D Code! | TCI Chemistry News October 2025 | [TCIPracticalExample] Di-Cbz Guanidinylation of Amine... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
请选择数量
CAS RN: 1883396-49-3 | 產品號碼: P2380
(S)-[4-(Pyridin-4-yl)-4,5-dihydro-3H-dinaphtho[2,1-c:1',2'-e]azepine-2,6-diyl]bis[bis[4-(tert-butyl)phenyl]methanol]
![(S)-[4-(Pyridin-4-yl)-4,5-dihydro-3H-dinaphtho[2,1-c:1',2'-e]azepine-2,6-diyl]bis[bis[4-(tert-butyl)phenyl]methanol] Chemical Structure of (S)-[4-(Pyridin-4-yl)-4,5-dihydro-3H-dinaphtho[2,1-c:1',2'-e]azepine-2,6-diyl]bis[bis[4-(tert-butyl)phenyl]methanol]](/medias/P2380.jpg?context=bWFzdGVyfHJvb3R8ODEzNTV8aW1hZ2UvanBlZ3xhRFprTDJnMU1pODRPVEl6TVRBeU1URTNPVEU0TDFBeU16Z3dMbXB3Wnd8NDBmMzQ5YmQ1MTEyM2JhZDk0Y2IzZjczYzk1MWRjZDY3MWE1YzEwY2VlNTBkMDE2ODQ0OTFmZmVjMjM4YzljOA)
* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | P2380 |
純度/分析方法 
|
>85.0%(HPLC) |
| 分子式 / 分子量 | C__6__9H__7__2N__2O__2 = 961.35 |
| 外觀與形狀(20°C) | Solid |
儲存條件 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 1883396-49-3 |
| PubChem Substance ID | 354333549 |
產品規格
| Appearance | Light orange to Yellow to Green powder to crystal |
| Purity(HPLC) | min. 85.0 area% |
| Elemental analysis(Nitrogen) | 2.50 to 3.30 % |
| Specific rotation [a]20/D | -270 to -310 deg(C=0.2, CHCl3) |
| NMR | confirm to structure |
性質
| 比旋光 [α]D | -290° (C=0.2,CHCl3) |
GHS
相關法規
運輸資料
| HS編碼* | 2933.39-000 |
Application
A Highly Active 4-Dimethylaminopyridine (DMAP) Derivative Chiral Nucleophilic Catalyst
These asymmetric organocatalysts [P2380] and [P2617] are novel chiral DMAP derivatives, developed by Mandai and Suga et al.1) They show extremely high catalytic activity (0.1-0.5 mol % catalyst) and enantioselectivity in chiral acylating reactions, such as Steglich rearrangements1), the kinetic resolution of secondary carbinols2) and D,L-1,2-diols3), and the desymmetrization of meso-1,2-diols.4)
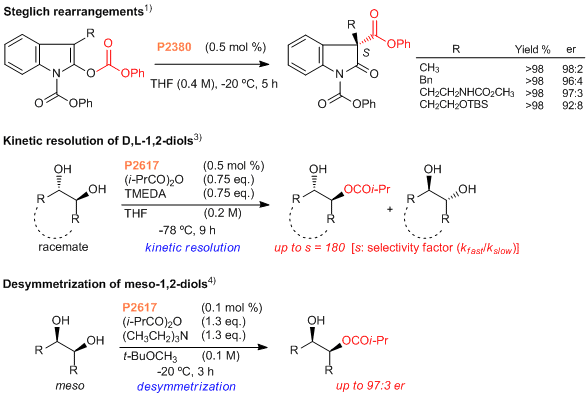
References
- 1)H. Mandai, K. Fujii, H. Yasuhara, K. Abe, K. Mitsudo, T. Korenaga, S. Suga, Nat. Commun. 2016, 7, 11297.

- 2)K. Fujii, K. Mitsudo, H. Mandai, S. Suga, Bull. Chem. Soc. Jpn. 2016, 89, 1081.

- 3)K. Fujii, K. Mitsudo, H. Mandai,, S. Suga, Adv. Synth. Catal. 2017, 359, 2778.

- 4)H. Mandai, H. Yasuhara, K. Fujii, Y. Shimomura, K. Mitsudo, S. Suga, J. Org. Chem. 2017, 82, 6846.

Application
Enantioselective Steglich Rearrangement with a Highly Active Chiral Nucleophilic Catalyst

Typical Procedure(Steglich rearrangement,1) R=CH3):
To a solution of phenyl 2-((phenoxycarbonyl)oxy)-3-methyl-1H-indole-1-carboxylate (37.4 mg, 0.1 mmol) in THF (0.25 mL) is added a solution of the DMAP chiral analog [P2380] (0.48 mg, 0.5 µmol) in THF at -20 °C. The reaction mixture was stirred for 5 h at -20 °C and then 1 M aqueous HCl is added. The resulting mixture is extracted with EtOAc, dried over MgSO4 and concentrated in vacuo. The purification of the crude product by flash column chromatography on a short pad of silica gel (eluent: hexane/Et2O = 1/1) gives (S)-diphenyl 3-methyl-2-oxoindoline-1,3-dicarboxylate (36.9 mg, 99% yield).
To a solution of phenyl 2-((phenoxycarbonyl)oxy)-3-methyl-1H-indole-1-carboxylate (37.4 mg, 0.1 mmol) in THF (0.25 mL) is added a solution of the DMAP chiral analog [P2380] (0.48 mg, 0.5 µmol) in THF at -20 °C. The reaction mixture was stirred for 5 h at -20 °C and then 1 M aqueous HCl is added. The resulting mixture is extracted with EtOAc, dried over MgSO4 and concentrated in vacuo. The purification of the crude product by flash column chromatography on a short pad of silica gel (eluent: hexane/Et2O = 1/1) gives (S)-diphenyl 3-methyl-2-oxoindoline-1,3-dicarboxylate (36.9 mg, 99% yield).
References
- 1)H. Mandai, K. Fujii, H. Yasuhara, K. Abe, K. Mitsudo, T. Korenaga, S. Suga, Nat. Commun. 2016, 7, 11297.

- 2)K. Fujii, K. Mitsudo, H. Mandai, S. Suga, Bull. Chem. Soc. Jpn. 2016, 89, 1081.

PubMed Literature
文檔
產品文件 (部分產品的分析圖譜無法提供,敬請諒解。)
SDS
請選擇語言。
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
請輸入批號
輸入的批號不正確
示例 CoA
可下載CoA示例。注:該示例可能非最新批次的CoA。
目前沒有該產品的 CoA 示例。
分析圖譜

請輸入批號
輸入的批號不正確
很抱歉,您搜索的分析圖譜無法提供。
![Thumbnail of (S)-[4-(Pyridin-4-yl)-4,5-dihydro-3H-dinaphtho[2,1-c:1',2'-e]azepine-2,6-diyl]bis[bis[4-(tert-butyl)phenyl]methanol]](/medias/P2380.jpg?context=bWFzdGVyfHJvb3R8MjA0Nzh8aW1hZ2UvanBlZ3xhR0ZsTDJnME9DODRPVE0yTlRBek16RXlOREUwTDFBeU16Z3dMbXB3Wnd8MjU3MTY1ZmRiYjBhZWJkZDg3NGViYjRjYTg2NmZkMDYyOGU4NDRkMWUwZDZlOTljMDExNzRjNzEzMThiYjQyYw)
![(S)-[4-(Pyridin-4-yl)-4,5-dihydro-3H-dinaphtho[2,1-c:1',2'-e]azepine-2,6-diyl]bis[bis[4-(tert-butyl)phenyl]methanol] (S)-[4-(Pyridin-4-yl)-4,5-dihydro-3H-dinaphtho[2,1-c:1',2'-e]azepine-2,6-diyl]bis[bis[4-(tert-butyl)phenyl]methanol]](/_ui/responsive/theme-tci/images/missing_product_zoom.webp)


