Maximum quantity allowed is 999
Please select the quantity
 | ||||
| TCI Chemistry News January 2021 | ||||
| TCI offers an extensive catalog of 30,000 high quality organic reagents suitable for benchtop-to-bulk chemistry. This issue covers our chemistry expertise in chiral sulfides for optically active three-membered rings, oxidizing agents, Williamson ether synthesis, and the latest issue of TCIMAIL. | ||||
 | ||||
Chiral Sulfides for Optically Active Three-membered Rings(-)-Isothiocineole [T2578] and (+)-isothiocineole [T2579] are limonene derivatives and useful chiral auxiliaries for the synthesis of optically active epoxides, aziridines and cyclopropanes. For instance, a sulfur ylide derived from T2578 reacts with aldehydes and tosylimides to afford chiral epoxides and aziridines, respectively, in high diastereo- and enantioselectivity. It is found that this asymmetric reaction can smoothly proceed on a gram scale. Another advantage is that both T2578 and T2579 can be recovered and recycled after the reaction. | ||||
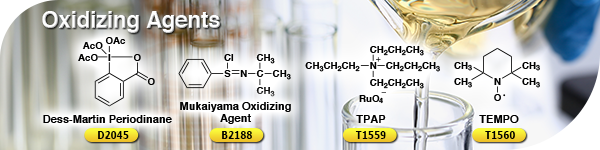 | ||||
Oxidizing AgentsOxidation, which makes its target substance lose electrons, is one of the most basic reactions in organic chemistry and is exemplified by the reaction with oxygen or a dehydrogenation reaction. In particular, oxidizing agents have often been used for the transformation of alcohols to the corresponding aldehydes, ketones or carboxylic acids. Less harmful oxidizing agents without heavy metals have been developed, such as Dess-Martin periodinane, the Mukaiyama oxidizing agent and oxoammonium salts. Moreover, oxidation reactions employing inexpensive cooxidants have been reported in the presence of oxidation catalysts like tetrapropylammonium perruthenate (TPAP) and TEMPO. | ||||
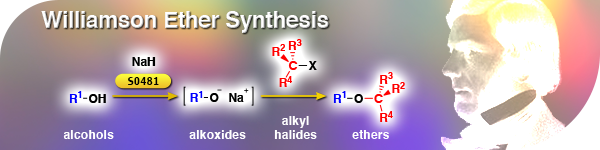 | ||||
Williamson Ether SynthesisThe Williamson ether synthesis is an old, but still frequently encountered organic reaction, which provides ether products most often from alcohols and alkyl halides. Alkoxides, derived from alcohols and bases such as sodium hydride are generated in situ, and alkyl halides can react through SN2 type mechanism to give a variety of ether products. The reactivity is generally dependent on the pertinent SN2 reaction, with steric being the dominant factor. | ||||
 | ||||
TCI Chemistry Research: TCIMAIL 185Released in November, TCI's latest issue features Assoc. Prof. Michael Schnürch's article on cross-coupling called "Sequential Triple Cross-Coupling towards Synthesis of 2,4,5-Trisarylthiazoles”. We also feature research on:
| ||||
| » TCI eNewsletter Back Issues | ||||
Follow Us on Social Media | ||||
|
How to Order TCI ProductsTo order, please contact your local distributor found below.» Our distributors |
| * The prices displayed in the landing website are USD based catalog prices. TCI has specific website and local currency based price list for customers in South Korea, Singapore, and Australia. For customers in other Asia Pacific countries / regions, please visit our Asia Pacific Website and contact our local distributors for the end user's cost. (The USD prices that are shown in Asia Pacific websites are TCI's catalog prices.) Please visit each country/region's website from the following links and confirm the prices. https://www.TCIchemicals.com |
|
If you are interested in our biweekly e-enewsletter "TCI News" and want to receive regularly, please click the link below and sign up. TCI News Sign-Up |
|
Tokyo Chemical Industry Co., Ltd. (TCI) Global Business Department Customer Support and Technical service » globalbusiness@TCIchemicals.com » https://www.TCIchemicals.com |





