Maintenance Notice (4:00 AM - 9:30 AM December 13, 11:30 PM December 13 - 1:40 AM December 14 2025): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.200
Maximum quantity allowed is 999
*Stock disponible en Belgique : Expédition le jour même
*Stock disponible au Japon : Veuillez consulter la simulation d'expédition pour une estimation des délais d'expédition (hors articles réglementés et expédition avec Carboglace)
This product has not been tested for potency and is guaranteed for chemical purity.
| Numéro de produit | E0751 |
Pureté / Méthode d'analyse 
|
>98.0%(T) |
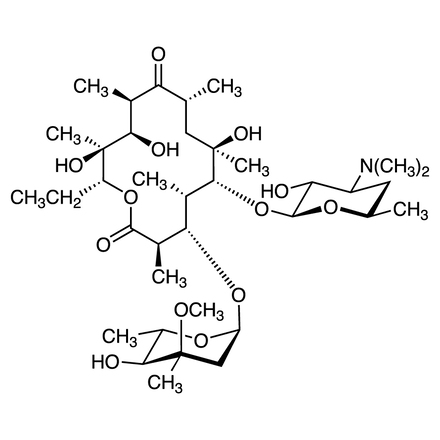
| Formule moléculaire / poids moléculaire | C__3__7H__6__7NO__1__3 = 733.94 |
| Etat physique (20 ° C) | Solid |
Condition de stockage 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| Stocker sous gaz inerte | Store under inert gas |
| Condition à éviter | Hygroscopic |
| CAS RN | 114-07-8 |
| Numéro de registre de Reaxys | 75279 |
| Identifiant de la substance PubChem | 87559996 |
| Indice Merck (14) | 3681 |
| Numéro MDL | MFCD00084654 |
| Appearance | White to Orange to Green powder to crystal |
| Purity(Nonaqueous Titration) | min. 98.0 %(calcd.on anh.substance) |
| Specific rotation [a]20/D | -71.0 to -78.0 deg(C=2, EtOH)(calcd.on anh.substance) |
| Water | max. 10.0 % |
| Rotation optique | -74° (C=2,EtOH) |
| Solubilité (soluble dans) | Ether, 1,2-Dichloroethane |
| Pictogramme |

|
| Mot de signal | Attention |
| Mentions de danger | H317 : Peut provoquer une allergie cutanée. |
| Conseils de prudence | P501 : Éliminer le contenu/récipient dans une installation d'élimination des déchets agréée. P261 : Éviter de respirer les poussières. P272 : Les vêtements de travail contaminés ne devraient pas sortir du lieu de travail. P280 : Porter des gants de protection. P362 + P364 : Enlever les vêtements contaminés et les laver avant réutilisation. P333 + P313 : En cas d'irritation ou d'éruption cutanée: consulter un médecin. |
| Numéros CE | 204-040-1 |
| RTECS # | KF4375000 |
| N ° SH (import / export) (TCI-E) | 2941500000 |
La FDS demandée n'est pas disponible.
Nous contacter pour plus d'informations.
Un échantillon CoA pour ce produit n'est pas disponible pour le moment.

Le tableau analytique demandé n'est pas disponible. Nous sommes désolés pour ce désagrément.