Maintenance Notice (4:00 AM November 1 - 9:30 AM November 1, 2025): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.199
Maximum quantity allowed is 999
We are proud to present the di-Boc guanidinylation of 4-bromophenethylamine using 1-[N,N'-(di-Boc)amidino]pyrazole as a guanidinylating agent.
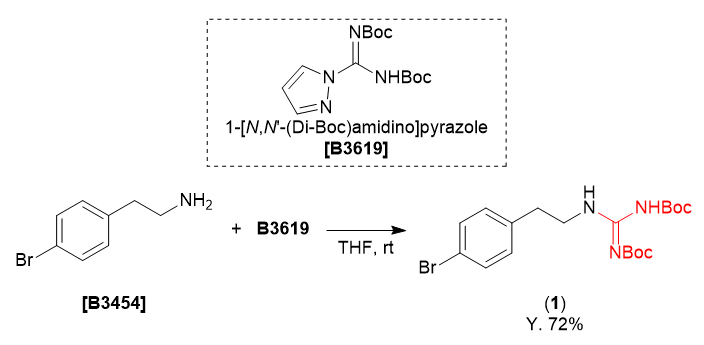
A solution of 4-bromophenethylamine (200 mg, 1.00 mmol) and 1-[N,N'-(di-Boc)amidino]pyrazole (341 mg, 1.10 mmol) in THF (1.7 mL) was stirred at room temperature for one day. The reaction mixture was concentrated under reduced pressure and the residue was purified by column chromatography (on silica gel, ethyl acetate:hexane = 0:100 - 10:90) to give the guanidinylated compound 1 as a pale yellow solid (319 mg, 72% yield.).
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 1:3, Rf = 0.55).
Guanidinylated Compound 1
1H NMR (270 MHz, DMSO-d6); δ 11.48 (s, 1H), 8.32 (t, J = 6.4 Hz, 1H), 7.48 (d, J = 8.1 Hz, 2H), 7.15 (d, J = 8.1 Hz, 2H), 3.51 (q, J = 6.4 Hz, 2H), 2.78 (t, J = 6.4 Hz, 2H), 1.47 (s, 9H), 1.40 (s, 9H)